Figure 4.
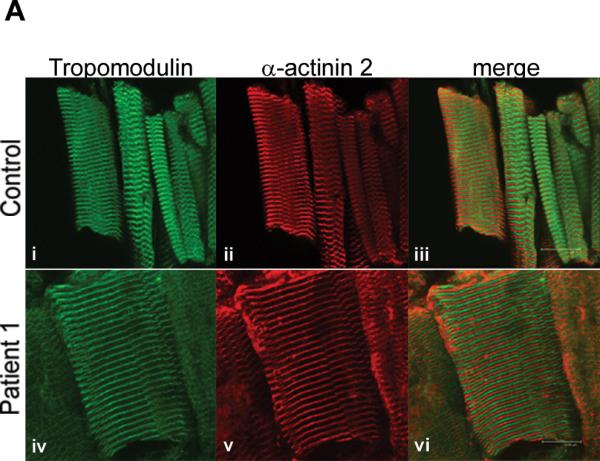
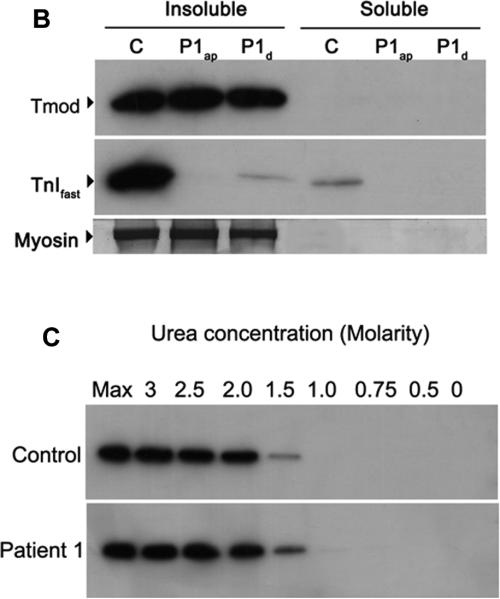
Tropomodulin is normally expressed and bound to the thin filament in a patient with a M9R mutation in TPM3. (A) Immunohistochemistry with tropomodulin shows a striated pattern in control (i, quadriceps muscle) and in Patient 1 (iv, adductor pollicis muscle). Labeling with α-actinin 2 also shows a striated pattern indicating its localization to the Z-line (ii) and (v). Tropomodulin does not colocalize with α-actinin 2 (iii and vi) due to its localization at the pointed end of the thin filament. The images were taken on a Leica SP2 confocal microscope at 60x magnification and 2.5x zoom. (B) Frozen muscle sections from Patient 1 (M9Rap = adductor pollicis, M9Rd = deltoid) and an age-matched control (quadriceps) were extracted into insoluble and soluble protein pools using 0.5% triton X-100 and separated by SDS-PAGE. Tropomodulin is expressed in the control and in both of the patient muscle samples in the insoluble pool. Troponin Ifast is detected at high levels in the control with only low levels in the patient samples. The PVDF membrane was stained with Coomassie blue myosin is shown to illustrate equal protein loading for each sample. (C) Myofibrillar proteins were extracted from frozen muscle sections from Patient 1 (adductor pollicis muscle) and an age matched control using 0.5% triton X-100 and denatured using varying concentrations of urea (0.5–3 M). The samples were solubilized, separated by SDS-PAGE and immunoblotted using anti-tropomodulin (Tmod#1749). In both the patient and the control samples the majority of tropomodulin (~80%) is denatured at 1.5 M urea, suggesting that there is no difference in the binding dynamics of tropomodulin to the thin filament.