Figure 4. The S54NVβ Mutation Modulates the Conformation of the Invariant Residue Glu56 to Facilitate an Extensive Water-Mediated Hydrogen Bonding Network with SEC3.
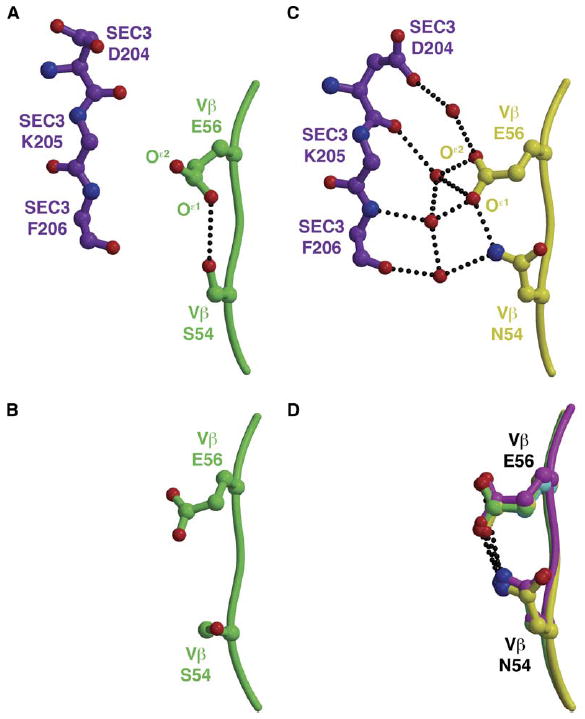
(A) An intramolecular hydrogen bonding interaction between S54Vβ and E56Vβ prevents interactions and intermolecular contacts with SEC3.
(B) The analogous region of the CDR2 loop of wild-type apo Vβ.
(C) The mutation S54NVβ extends both N54Vβ and E56Vβ side chains toward SEC3, resulting in the recruitment of numerous ordered water molecules to the molecular interface.
(D) The relative conformations of the N54Vβ and E56Vβ side chains are ordered prior to binding SEC3, as seen in all apo Vβ variants containing the A52VVβ mutation for which we have determined crystal structure. Hydrogen bonds are shown as black, dotted lines.
