Figure 6. Molecular Interplay between Variant Residues at Positions 52Vβ and 72Vβ Affects the Vβ CDR1 Loop.
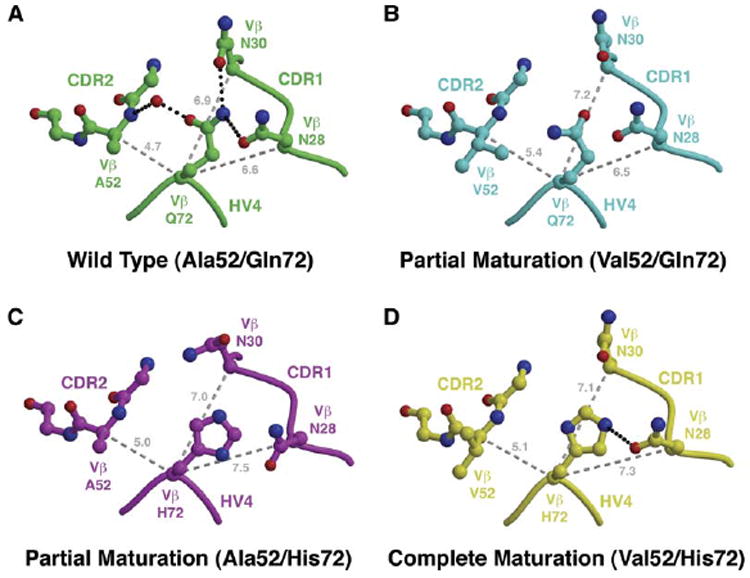
(A–D) Comparison of CDR1/CDR2/HV4 loop arrangements in apo Vβ structures containing (A) both wild-type A52Vβ and Q72Vβ residues, (B) the A52VVβ mutation and the wild-type Q72Vβ residue, (C) the wild-type A52Vβ residue and the Q72HVβ mutation, and (D) both A52VVβ and Q72HVβ mutations. Hydrogen bonds are shown as black, dotted lines. Distances (in Å) between the Cα atom of position 72Vβ and the Cα atoms of positions 28Vβ, 30Vβ, and 52Vβ are shown as gray, dashed lines.
