Fig. 2.
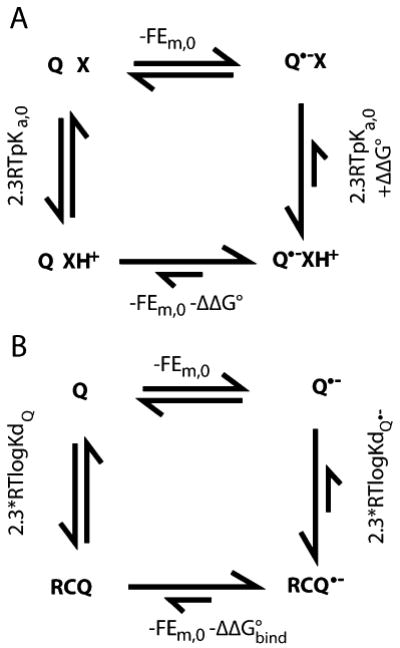
Modification of quinone Ems by proton or protein binding. a Energetics of coupled electron and proton transfer. X is a base associated with a quinone binding site. When X is neutral the Em for Q is Em,0; when Q is neutral the pKa for X is pKa,0. Favorable interactions between X+ and Q•− raise the Em for Q by −ΔΔG°/nF. The same interaction stabilizes XH+, raising the pKa by ΔΔG°/2.3RT. A ΔΔG° of −1.36 kcal/mol shifts the Em up by 58 mV and the pKa up by 1 pH unit. If the pKa of X remains below the pH in the presence of Q•− then X will never be protonated and Em=Em,0. If the pH is below the pKa of X with Q oxidized, XH+ will be present throughout the reaction and the observed Em will be Em=Em,0−ΔΔG°/F. In this case the reduction of Q feels the full stabilization by the adjacent base. In either case the reaction is pH independent. However, if the pH is at least ≈2 pH units below the pKa with Q and ≈2 pH units above the pKa in the presence of Q•− (i.e. ΔΔG° >≈5.4 kcal/mol) then binding ≈1 proton will be coupled to electron transfer (when the pH is 2 pH units below the pKa X will remain 1% protonated; when the pH is 2 pH units above the pKa X will remain 1% XH+. Thus, on average 0.98 more protons will be bound). The resultant Em will be Em,0− [ΔΔG°+(pH−pKa,0)/2.3RT]/F. The free energy needed to protonate X at this pH is (pH−pKa,0)/2.3RT. It is this term which leads to the classic 60 meV/pH unit Em shift with pH indicative of 1 proton bound/electron. The cost of rearranging the surroundings diminishes the Em shift from that found if XH+ were present at the start of the reaction. If pKa,0 for X is near the pH even a small ΔΔG° of interaction with a more distant Q•− will lead to substoichiometric changes in protonation of X, leading to a pH dependence smaller then 60 mV/pH unit. b The relationship between the thermodynamics of quinone binding and quinone electrochemistry. If the Em is more positive in the quinone binding site then the semiquinone is bound more tightly then the quinone. ΔΔG°bind is 2.3RT(logKdQ•−− logKdQ)
