Fig. 4.
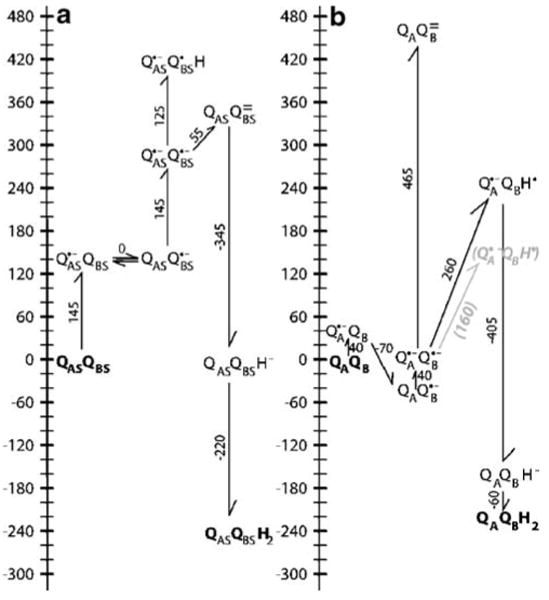
Energy levels for sequential electron transfer from a primary semiquinone to a secondary quinone. A QAS:QBS indicate a complex of two quinones which have the same electrochemistry as two isolated ubiquinones in solution at pH 7 (Fig. 1). To start the cycle one quinone is reduced to the semiquinone forming QAS•−:QBS. Given a solution Eh of 0 mV or a chemical electron donor with an Em of 0 mV, the single reduction of quinone with an Em of −145 mV to Q•− is uphill by 145 meV. Since these quinones are identical, electron transfer from QAS•− to QBS will be isoenergetic. Given the semiquinone pKa of 4.9, protonating either semiquinone would be uphill by ≈120 meV. Thus, the lowest energy, singly reduced state will be a 50:50 mixture of QAS•−:QBS and QAS:QBS•−. The second turnover starts with the second reduction of QAS forming QAS•−:QBS•−. The thermodynamically preferred pathway has the electron transfer occurring before the first proton is bound. The formation of QAS:QBS= requires only 55 meV because the second reduction of QBS is coupled to the favorable oxidation of QAS. The reaction path where QBS•− is protonated to form QAS•−:QBSH before it is reduced is 120 meV uphill. QAS:QBSH− where one quinone has two electrons and one proton is 290 meV lower in energy then QAS•−:QBS•− and is essentially isoenergetic with the initial QAS:QBS state at pH 7. Once QAS:QBSH− is formed the second protonation to form QAS:QBSH2 is downhill by −220 meV. B The ubiquinone energy levels in R. sphaeroides RCs at pH 7 and Eh 0. In the initial reaction QA is reduced to the semiquinone forming QA•−:QB. Calculations put this state near 0 mV, close to the measured values between −45 and −70 mV (all calculated values are from Zhu and Gunner (2005)). QB•− is stabilized by 30 (calculation) to ≈70 meV more then QA•−. The second turnover starts with formation of QA•−:QB•−. Calculations and the experiments of Graige and Okamura show that in the protein QA•−:QB•H is lower in energy then QA:QB= (Graige et al. 1998). The calculations place the energy of QA•−:QB•H 260 meV above QA•−:QB•−, while the kinetics of forward electron transfer support a value of 160 meV (grey text; Graige et al. 1999). The second reduction of QBH− is favorable. The anionic QAQBH− is 110 meV more stable in the protein then in solution, while QAQBH2 is 50 meV less stable favoring quinol dissociation
