Abstract
Aims and Hypothesis
Circulating β-carotene levels are inversely associated with type 2 diabetes risk, but the causal direction of this association is not certain. In this study we used a Mendelian Randomization approach to provide evidence for or against the causal role of the anti-oxidant vitamin β-carotene in type 2 diabetes.
Methods
We used a common polymorphism (rs6564851) near the β-carotene 15,15'-Monooxygenase 1 (BCMO1) gene that is strongly associated with circulating β-carotene levels (P = 2×10−24) - each G allele is associated with a 0.27 standard deviation increase in levels. We used data from the InCHIANTI study and the ULSAM study to estimate the association between β-carotene levels and type 2 diabetes. We next used a triangulation approach to estimate the expected effect of rs6564851 on type 2 diabetes risk, and compared this to the observed effect using data from 4549 type 2 diabetes cases and 5579 controls from the DIAGRAM consortium.
Results
A 0.27 standard deviation increase in β-carotene levels is associated with an odds ratio of 0.90 (0.86–0.95) for type 2 diabetes in the InCHIANTI study. This association is similar to that of the ULSAM study, OR (0.90 (0.84–0.97)). In contrast there was no association between rs6564851 and type 2 diabetes (OR 0.98 (0.93–1.04, P = 0.58), and this effect size was smaller than that expected given the known associations between rs6564851 and β-carotene levels and the associations between β-carotene levels and type 2 diabetes.
Conclusion
Our Mendelian Randomization studies are in keeping with randomized controlled trials that suggest β-carotene is not causally protective against type 2 diabetes.
Keywords: type 2 diabetes, β-carotene, mendelian randomization
Introduction
Circulating β-carotene levels are associated with type 2 diabetes, but the causal direction of this association is disputed. Recently, Ärnlöv et al reported results of a longitudinal community-based study, Uppsala Longitudinal Study of Adult Men (ULSAM), assessing risk of serum and dietary β-carotene on the incidence of type 2 diabetes1. The ULSAM study observed a strong association between increased baseline serum levels at age 50 years and reduced type 2 diabetes incidence during 27 years of follow up. For a 1 standard deviation (SD) increase in serum β-carotene, they observed a protective effect with an odds ratio (OR) of 0.68 (0.53–0.89). The authors also reported that a 1SD increase in β-carotene levels at age 50 years was associated with improved insulin sensitivity at aged 70 years, in non-diabetic individuals. Ärnlöv et al argued that these associations support the importance of impaired antioxidant status for the development of insulin resistance and type 2 diabetes. They also suggested that antioxidants could be involved early in the pathological processes leading to diabetes and that it takes a long period of exposure to low antioxidant levels before metabolic factors are affected. These findings are consistent with some but not all observational epidemiological reports on the role of β-carotene levels in type 2 diabetes. Previous studies have provided evidence that β-carotene is not causally associated with type 2 diabetes2,3. Notably three placebo controlled trials, the Physicians' Health Study4, the Alpha-Tocopherol, Beta-Carotene Cancer Prevention (ATBC) Study5 and the Women's Antioxidant Cardiovascular Study6 have all reported null effects of β-carotene supplementation on adverse metabolic effects including type 2 diabetes.
A caveat to observational epidemiological studies is that associations between risk factors and disease incidence many years later do not necessarily strengthen the case that the risk factor is causal. Disease processes can begin many years before disease diagnosis, and adverse metabolic effects have been reported as early as the first decade of life7. Confounding factors may also result in a misleading association between anti-oxidant vitamins and adverse metabolic outcomes such as diabetes. We note that the association between β-carotene levels and type 2 diabetes in the ULSAM study was stronger before correcting for BMI, self reported physical activity and smoking status1.
Genetics studies may be able to help dissect the causal directions of disease-biomarker associations. Genotypes cannot be influenced by disease status or any other trait, therefore making them much less likely to be confounded or the result of reverse causation than non-genetic factors. This principle of `Mendelian Randomization' (MR) has been applied before to indicate that C-Reactive Protein is unlikely to have a causal role in the development of various metabolic traits8. More recently it has also been applied to examine the role between a range of inflammatory proteins and type 2 diabetes. Rafiq et al found no evidence for a causal role of inflammatory or autoimmune factors on type 2 diabetes risk, including Interleukin 189.
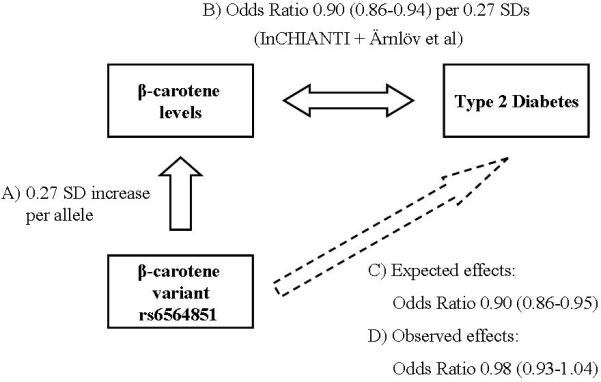
We have used a Mendelian Randomization approach to help dissect the causal role of β-carotene in type 2 diabetes risk (Figure 1). To do this we used a) a common polymorphism (rs6564851) near the β-carotene 15,15'-Monooxygenase 1 (BCMO1) gene recently identified as strongly associated with circulating β-carotene levels; b) an estimate of the association between β-carotene levels and type 2 diabetes using two studies; c) an estimate of the expected effect of rs6564851 on type 2 diabetes risk given a) and b); and finally d) a large case control study to assess the observed effect of the β-carotene-associated SNP on type 2 diabetes.
Figure 1.
Triangulation of β-carotene levels and risk of type 2 diabetes
Associations between the SNP rs6564851 and β-carotene levels; β-carotene levels and type 2 diabetes; the expected and observed effects of rs6564851 on type 2 diabetes. Odds Ratios for the β-carotene – type 2 diabetes association are estimated for a 0.27 SD increase in β-carotene. Odds ratios are shown with 95% CIs.
Methods
SNP - β-carotene association
We recently reported results from a Genome-Wide Association (GWA) study, that identified a polymorphism near the β-carotene 15,15'-Monooxygenase 1 (BCMO1) gene as robustly associated with fasting serum β-carotene levels (rs6564851, P = 2×10–24, 0.15 mmol/liter per-allele effect)10. The finding was consistent across three studies including individuals from across the adult age range. Using discovery and replication data combined, each G allele at rs6564851 was associated with a 0.27 SD increase in β-carotene levels.
β-carotene - type 2 diabetes association
To obtain an estimate of the association between circulating β-carotene and type 2 diabetes we used data from Ärnlöv et al and unpublished data from the InCHIANTI study11. For InCHIANTI, age and sex adjusted Z-scores were produced for fasting serum β-carotene levels (N = 1191). Of these 1191 individuals, 112 had clinically defined type 2 diabetes. Linear regression was used to calculate a per-allele additive effect on β-carotene levels. Within the InCHIANTI cohort, a 1SD increase in circulating β-carotene was associated with reduced type 2 diabetes risk OR 0.68 (95% C.I 0.56–0.82). This was similar to the findings of Ärnlöv et al in their combined (lifestyle and metabolic covariates) model OR 0.68 (0.53–0.89).
Estimated SNP - type 2 diabetes association
Given a) and b) we calculated that, if circulating β-carotene levels were causally involved in type 2 diabetes, a SNP with a 0.27SD increase in circulating levels should give a reduced type 2 diabetes risk of approximately an Odds Ratio of 0.90 (0.86–0.94). These estimated OR and confidence intervals were calculated by meta-analysis of the two effect estimates of a 1 SD increase in B-carotene levels on Type 2 diabetes risk from the InCHIANTI and ULSAM studies. To estimate a 0.27 SD effect, we then multiplied 0.27 by the 1SD OR effect sizes on the natural log scale (e.g 0.27*ln(0.68) = 0.90).
Observed SNP - type 2 diabetes association
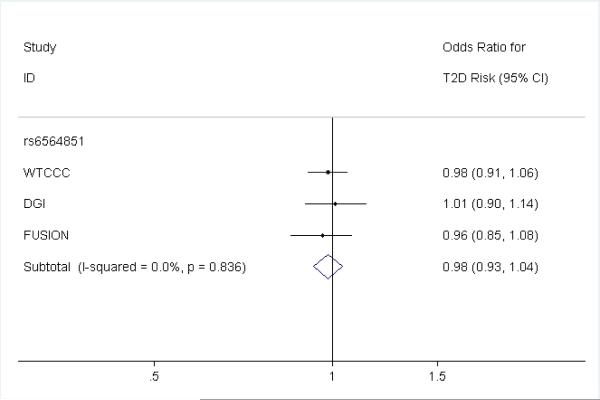
We used data from the published dataset of 4549 type 2 diabetes cases and 5579 controls from the DIAGRAM consortium12 to calculate an observed effect of rs6564851 on type 2 diabetes risk (detail of cases and controls in Supplementary Table 1). Within the DIAGRAM meta-analysis data, rs6564851 was directly genotyped in 1 / 3 studies (FUSION), and passed all imputation-QC criteria (MAF ~ 45%, DGI / WTCCC r2hat 0.76 / 0.96 respectively) in the 2 studies which imputed it.
Results
We did not observe any association between the β-carotene SNP rs6564851 and type 2 diabetes risk (OR 0.98 (0.93–1.04, P = 0.58)). Each β-carotene raising allele of the SNP was associated with a point estimate effect size (OR 0.98) outside of the predicted effect range from the circulating levels estimate (0.86–0.94). The individual effect estimates for each of the three DIAGRAM studies is presented in figure 2.
Figure 2.
Three study DIAGRAM results for rs6564851 on type 2 diabetes risk
Odds Ratio effect based on per β -carotene raising G allele.
Discussion
Our data provide evidence that exposure to life-long, modestly lower β-carotene levels does not increase the risk of type 2 diabetes. Our results are in keeping with the negative results from randomized controlled trials4–6. We suggest that the associations between β-carotene and type 2 diabetes are more likely to be confounded or the consequence of diabetes disease processes rather than aetiological. It is well accepted that observational epidemiological studies can be confounded even when they account for multiple covariates. Imperfect measurement of known, and no measurement of unknown, confounding factors can often result in spurious associations. It is also now well known that disease processes can begin long before diagnosis and metabolic disease processes clearly cause many secondary metabolic changes. A build up of disease processes over many years could mean that long term prospective studies are not immune from reverse causation. These factors could explain the difference in results between many of the observational epidemiology studies13–16 and the randomized controlled trials and genetic studies.
There are limitations to our Mendelian randomization approach17. The main one is that the approach tests the effects of life long altered exposure to modest differences in levels which could mean the body adapts early to the altered state and it has no adverse effect. Studies of common gene variants that alter LDL cholesterol suggest this is not necessarily a concern - common gene variants with subtle, life-long effects on LDL-cholesterol also alter the risk of coronary heart disease18,19. It is also important to note that the weakness of a Mendelian randomization approach may also be a strength, depending on the disease mechanism - Mendelian randomization is likely to be testing the effects of small changes over a longer time compared to randomized controlled trials that compare the effects of a larger change over a much shorter time. It is also possible that altered intra-cellular levels, which are not accounted for by Mendelian randomization approaches could have a disease effect. A further limitation is that the association between β-carotene levels and Type 2 diabetes is based on a relatively small number of cases and controls with wide confidence intervals. However, the fact that two studies have very similar results suggests that the point estimate of the uncorrected association between β-carotene levels and type 2 diabetes is a good approximation of the real whole-population association.
Conclusion
We suggest that the associations between β-carotene and type 2 diabetes are more likely to be confounded or the consequence of diabetes processes rather than aetiological. A combination of randomized supplementation trials and Mendelian randomization studies together provide a powerful argument that the anti-oxidant β-carotene is unlikely to be causally involved in the pathogenesis of type 2 diabetes.
Supplementary Material
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Institute on Aging
Abbreviations
- (GWA)
Genome-wide Association
- (OR)
Odds Ratio
- (MR)
Mendelian Randomization
References
- 1.Arnlov J, et al. Serum and dietary beta-carotene and alpha-tocopherol and incidence of type 2 diabetes mellitus in a community-based study of Swedish men: report from the Uppsala Longitudinal Study of Adult Men (ULSAM) study. Diabetologia. 2009;52:97–105. doi: 10.1007/s00125-008-1189-3. [DOI] [PubMed] [Google Scholar]
- 2.Kataja-Tuomola M, et al. Effect of alpha-tocopherol and beta-carotene supplementation on the incidence of type 2 diabetes. Diabetologia. 2008;51:47–53. doi: 10.1007/s00125-007-0864-0. [DOI] [PubMed] [Google Scholar]
- 3.Reunanen A, Knekt P, Aaran RK, Aromaa A. Serum antioxidants and risk of non-insulin dependent diabetes mellitus. Eur J Clin Nutr. 1998;52:89–93. doi: 10.1038/sj.ejcn.1600519. [DOI] [PubMed] [Google Scholar]
- 4.Liu S, et al. Long-term beta-carotene supplementation and risk of type 2 diabetes mellitus: a randomized controlled trial. Jama. 1999;282:1073–5. doi: 10.1001/jama.282.11.1073. [DOI] [PubMed] [Google Scholar]
- 5.The ATBC Cancer Prevention Study Group The alpha-tocopherol, beta-carotene lung cancer prevention study: design, methods, participant characteristics, and compliance. Ann Epidemiol. 1994;4:1–10. doi: 10.1016/1047-2797(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 6.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and {beta}-carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009 doi: 10.3945/ajcn.2009.27491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Whincup PH, et al. Early evidence of ethnic differences in cardiovascular risk: cross sectional comparison of British South Asian and white children. Bmj. 2002;324:635. doi: 10.1136/bmj.324.7338.635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Timpson NJ, et al. C-reactive protein and its role in metabolic syndrome: mendelian randomisation study. Lancet. 2005;366:1954–9. doi: 10.1016/S0140-6736(05)67786-0. [DOI] [PubMed] [Google Scholar]
- 9.Rafiq S, et al. Gene variants influencing measures of inflammation or predisposing to autoimmune and inflammatory diseases are not associated with the risk of type 2 diabetes. Diabetologia. 2008;51:2205–13. doi: 10.1007/s00125-008-1160-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrucci L, et al. Common variation in the beta-carotene 15,15'-monooxygenase 1 gene affects circulating levels of carotenoids: a genome-wide association study. Am J Hum Genet. 2009;84:123–33. doi: 10.1016/j.ajhg.2008.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrucci L, et al. Subsystems contributing to the decline in ability to walk: bridging the gap between epidemiology and geriatric practice in the InCHIANTI study. J Am Geriatr Soc. 2000;48:1618–25. doi: 10.1111/j.1532-5415.2000.tb03873.x. [DOI] [PubMed] [Google Scholar]
- 12.Zeggini E, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–45. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coyne T, et al. Diabetes mellitus and serum carotenoids: findings of a population-based study in Queensland, Australia. Am J Clin Nutr. 2005;82:685–93. doi: 10.1093/ajcn.82.3.685. [DOI] [PubMed] [Google Scholar]
- 14.Hozawa A, et al. Associations of serum carotenoid concentrations with the development of diabetes and with insulin concentration: interaction with smoking: the Coronary Artery Risk Development in Young Adults (CARDIA) Study. Am J Epidemiol. 2006;163:929–37. doi: 10.1093/aje/kwj136. [DOI] [PubMed] [Google Scholar]
- 15.Montonen J, Knekt P, Jarvinen R, Reunanen A. Dietary antioxidant intake and risk of type 2 diabetes. Diabetes Care. 2004;27:362–6. doi: 10.2337/diacare.27.2.362. [DOI] [PubMed] [Google Scholar]
- 16.Ylonen K, et al. Dietary intakes and plasma concentrations of carotenoids and tocopherols in relation to glucose metabolism in subjects at high risk of type 2 diabetes: the Botnia Dietary Study. Am J Clin Nutr. 2003;77:1434–41. doi: 10.1093/ajcn/77.6.1434. [DOI] [PubMed] [Google Scholar]
- 17.Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med. 2008;27:1133–63. doi: 10.1002/sim.3034. [DOI] [PubMed] [Google Scholar]
- 18.Kathiresan S, et al. Polymorphisms associated with cholesterol and risk of cardiovascular events. N Engl J Med. 2008;358:1240–9. doi: 10.1056/NEJMoa0706728. [DOI] [PubMed] [Google Scholar]
- 19.Willer CJ, et al. Newly identified loci that influence lipid concentrations and risk of coronary artery disease. Nat Genet. 2008;40:161–9. doi: 10.1038/ng.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.