Abstract
Purpose
The effects of omeprazole on indinavir when administered alone or in combination with ritonavir were evaluated.
Methods
Fourteen men and women age 18–55 years not infected with human immunodeficiency virus who met study qualifications were randomized to receive placebo, 20 mg of omeprazole, or 40 mg of omeprazole daily. After seven days, the single-dose pharmacokinetic profile of an 800-mg dose of indinavir alone or in combination with 200 mg of ritonavir was evaluated. Study participants received each of four study regimens in one of four randomly assigned orders. Blood samples were collected, and plasma indinavir and ritonavir concentrations were analyzed using high-performance liquid chromatography.
Results
The coadministration of 20 or 40 mg of omeprazole with indinavir significantly reduced the mean indinavir area under the concentration-versus-time curve (AUC) from 30.0 mg · hr/L (95% confidence interval [CI], 21.9–41.1 mg · hr/L) to 19.7 mg · hr/L (95% CI, 14.6–26.8 mg · hr/L) or 16.0 mg · hr/L (95% CI, 11.8–21.7 mg · hr/L), respectively (p < 0.002). The addition of 200 mg of ritonavir to 800 mg of indinavir in combination with 40 mg of omeprazole significantly increased the mean indinavir AUC from 30.0 mg · hr/L (95% CI, 21.9–41.1 mg · hr/L) to 46.6 mg · hr/L (95% CI, 34.0–63.8 mg · hr/L), but it did not significantly affect mean omeprazole concentrations (p ≤ 0.02).
Conclusion
The AUC of indinavir was substantially decreased in healthy volunteers who received omeprazole 20 or 40 mg daily for seven days before the administration of a single 800-mg dose of indinavir. Concomitant administration of ritonavir 200 mg with indinavir in participants receiving omeprazole led to a significant increase in the AUC of indinavir.
Index terms: Antiretroviral agents, Blood levels, Dosage, Drug interactions, Gastrointestinal drugs, HIV infections, Indinavir, Omeprazole, Pharmacokinetics, Ritonavir
Protease inhibitors (PIs) exhibit a high degree of pharmacokinetic variability in patients infected with the human immunodeficiency virus (HIV).1,2 Large interindividual differences in drug absorption and elimination in HIV-infected patients have been primarily attributed to constitutive or altered drug metabolizing enzymes, P-glycoprotein transporter activities, and poor drug solubility.3 With the PIs indinavir and atazanavir, changes in gastric pH can alter drug absorption.4,5 Specifically, when these PIs are administered with medications that increase gastric pH, such as histamine (H2)-receptor antagonists and proton-pump inhibitors (PPIs), bioavailability can decrease by up to 76%.4,6
A survey of 200 HIV-infected patients was performed to assess their use of drugs that affect gastric acidity.7 Fifty-six percent of HIV-infected patients who had recently begun highly active antiretroviral therapy (HAART) had taken nonprescription acid-reducing agents, and 39% had used both nonprescription and prescription products for acid reduction. Forty-six percent of patients on a PI-containing regimen had used PPIs or H2-receptor antagonists once they started HAART, and 35% had used them within the previous 12 months. This widespread use of acid-reducing agents among HIV-infected patients has implications for drug interactions with antiretrovirals and other medications that require an acidic environment for adequate dissolution and absorption.
Ritonavir is a cytochrome P-450 (CYP) isoenzyme 3A and P-glycoprotein inhibitor which, when used in low doses, can increase the exposure of concomitantly administered PIs. The concomitant administration of ritonavir with atazanavir to enhance the absorption of atazanavir when used concurrently with acid-reducing agents has been investigated.7 While these studies show that concomitant administration of ritonavir does not adequately increase atazanavir exposure in the presence of PPIs, it does maintain adequate atazanavir exposure in the presence of H2-receptor antagonists.4,8
The dissolution and absorption of indinavir are also dependent on an acidic environment.5 Due to the paucity of data on indinavir combined with omeprazole, we conducted a prospective, randomized, placebo-controlled, single-dose crossover study to evaluate the influence of two different doses of omeprazole on indinavir pharmacokinetics.
Methods
Participants
Sixteen HIV-uninfected men and women ages 18 to 55 years were recruited for this study, which was approved by the institutional review board of the University of North Carolina (UNC) at Chapel Hill, School of Medicine. Informed consent was obtained from all volunteers before study screening. Women of childbearing age were required to have a negative serum or urine β-human chorionic gonadotropin test at screening. All participants were tested for HIV antibodies using the standard blood test procedure (enzyme-linked immunosorbent assay [ELISA] plus West blot). Participants were excluded if they had any of the following disorders: active gastrointestinal disease (including, but not limited to, peptic ulcer disease and gastroesophageal reflux disease), liver disease based on laboratory test results (aspartate transaminase, alanine transaminase, or a total bilirubin level of >3.0 times the upper limit of normal), prior allergy or intolerance to any study medication, renal disease (serum creatinine of ≥1.5 mg/dL), a history of nephrolithiasis, or active drug or alcohol abuse or dependence that would interfere with adherence to study requirements. Concomitant use of other medications known to influence CYP, drug transporter activity, or gastric pH was not permitted.
Study design
Participants were assigned by means of a permuted block randomization algorithm into one of four groups. Each group completed four visits. The patients were randomized to receive seven days of placebo daily (visit A), 20 mg of omeprazole daily (visit B), or 40 mg of omeprazole daily (visits C and D). After seven days, the single-dose pharmacokinetics of an 800-mg dose of indinavir (Crixivan, Merck & Co., Inc., Whitehouse Station, NJ) (visits A, B, and C) or indinavir 800 mg administered in combination with 200 mg of ritonavir (Norvir, Abbott Laboratories, Abbott Park, IL) (visit D) was evaluated. Each visit was separated by at least a seven-day washout period to allow for the elimination of omeprazole and to avoid treatment-by-period interactions. Participants were given placebo and a single dose of indinavir for period A, omeprazole 20 mg and a single dose of indinavir for period B, omeprazole 40 mg and a single dose of indinavir for period C, and omeprazole 40 mg and a single dose of indinavir plus ritonavir for period D. Participants received each of the four study regimens in one of four randomly assigned orders: Group 1—A, D, C, B; Group 2—B, C, D, A; Group 3—C, B, A, D; and Group 4—D, A, B, C. Before the morning of the seventh day of each visit, patients were admitted to the General Clinical Research Center at UNC Hospitals. A complete physical examination was performed, and laboratory test results (blood chemistry profiles, liver function tests, and complete blood counts with differential) were obtained. The occurrence of study-related adverse effects was assessed at each visit by study personnel and graded according to the adult AIDS Clinical Trials Group (ACTG) criteria.9
At 8:00 on the morning of the 7th, 21st, 35th, and 49th days, patients received either 800 mg of indinavir orally or 800 mg of indinavir with 200 mg of ritonavir orally in addition to omeprazole or placebo. Participants receiving indinavir without ritonavir were given a low-fat breakfast (446 kcal, 3.2 g fat, 12.2 g protein, and 95.2 g carbohydrates) one hour after indinavir was administered. Participants receiving indinavir with ritonavir were given a standardized, normal meal (905 kcal, 31.9 g fat, 33 g protein, and 116.7 g carbohydrates) at the time of medication administration. The drugs were administered with meals based on the manufacturer’s dosing recommendations.6 Since participants received single doses of indinavir, we did not deem them at risk for nephrolithiasis; thus, participants were not required to drink ≥1.5–2 L of liquid per day when taking indinavir, as recommended by the manufacturer.
Blood sampling was performed over 24 hours after the observed administration of the indinavir or indinavir and ritonavir doses at the following intervals: 0, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 18, and 24 hours. A total of 10 mL of blood was collected in tubes containing tripotassiumethylenediaminetetraacetic acid as the anticoagulanta and centrifuged for 15 minutes at 3000 rpm at 2 °C. Four 1-mL cryogenic vials were filled with plasma supernatant and stored temporarily at −20 °C until all samples from each visit had been collected. Samples were then transferred to a −70 °C freezer until analysis.
All study medications were prepared and dispensed by the hospital’s investigational drugs pharmacy. Doses were prepared in nontransparent, darkly colored capsules containing either 20 or 40 mg of omeprazole or placebo tablets containing lactose, steric acid, and magnesium stearate. Medication adherence was assessed at each study visit by pill count or direct observation by research staff.
Sample analyses
Plasma indinavir and ritonavir concentrations were analyzed according to a high-performance liquid chromatography (HPLC) method with ultraviolet detection as described by Rezk and colleagues.10 Briefly, a standard stock solution of indinavir and ritonavir was prepared at a 1-mg/mL concentration in composite with other PIs and nonnucleosides. A 550-μL plasma sample was mixed with 550 μL of 125-mM ammonium acetate containing an internal standard (midazolam) and subjected to solid-phase extraction using BOND ELUT-C18 columns (1.0 mL, 100 mg)b The eluted samples were evaporated and reconstituted in 100 μL of mobile phase before injection into the HPLC system.c The mobile phase consisted of (A) 50 mM of phosphate monobasic (pH 4.5) 85% mixed with methanol 15% and (B) 250 mL of buffer as mobile phase A, mixed with 600 mL of acetonitrile, 150 mL of methanol, and 0.75 mL of trifluoroacetic acid. Chromatographic separation was accomplished with an analytical column (3.5 μm, 150 × 4.6 mm)d and a guard column (3.5 μm, 12.5 × 4.6 mm).e Separation was facilitated via a linear-gradient elution of mobile phase as 36–86% of mobile phase (B) and a mobile-phase flow rate gradient of 0.9–1.2 mL over 30 minutes. Calibration standard curves ranged from 10 to 10,000 ng/mL. Intraday and interday variations for indinavir and ritonavir were <2.3% and <1.2%, respectively.
Plasma extraction and detection of omeprazole were adapted from a previously published method.11 Briefly, 200 ng of internal standard, H153/52,f 400 μL of 0.5 M potassium phosphate buffer solution (pH 8.0), 50 mg of sodium chloride, and 1.5 mL of dichloromethane were added to 1 mL of plasma. Samples were vortexed and centrifuged, and the lower dichloromethane layer was evaporated to dryness under nitrogen at room temperature. Samples were reconstituted in 150 μL of mobile phase, and 100 μL of that solution was injected onto a CAPCELL PAK UG 120 (2 × 250 mm) 5-μm particle-size column.g The mobile phase consisted of acetonitrile with 0.05 M sodium phosphate buffer (pH 8.5) (27:73, by volume) delivered at a flow rate of 0.3 mL/min. The detection wavelength was fixed at 302 nm. Integration was performed using HP ChemStation software.h Standard curves were linear and reproducible over the concentration range of 100–10,000 ng/mL. Intraday and interday variability was ≤15%.
Data analysis
The pharmacokinetic parameters for indinavir and ritonavir were evaluated from plasma drug concentration data using a non-compartmental analysis.i Indinavir’s and omeprazole’s maximum plasma concentration (Cmax) and time to Cmax (tmax) were obtained from direct observation of the data. The area under the concentration-versus-time curve from zero to 24 hours (AUC0-24) was calculated using the linear trapezoidal rule.
A linear mixed-effect model with a compound symmetry covariance structure was used to compare the AUCs of indinavir and omeprazole, and a regression analysis was performed comparing omeprazole AUCs and indinavir exposures.j The study sample size was calculated to have 90% power to detect a 50% decrease in indinavir AUC. All data were expressed as the geometric mean (95% confidence interval [CI]) unless otherwise stated.
Results
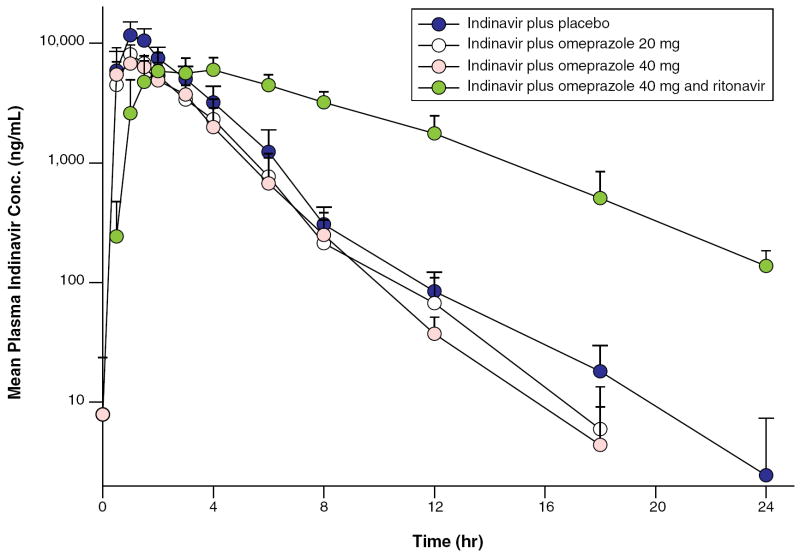
Sixteen volunteers were screened for enrollment and 14 participants completed all four visits; two participants were excluded due to their difficulty in obtaining an i.v. access and their inability to swallow capsules. Of those remaining 14 participants, 7 were men, 11 were Caucasian, 2 were African American, and 1 was Hispanic. The mean age for these participants was 32 years (range, 23–59 years). No severe adverse events occurred during the study. Adverse events included ACTG criteria grade 1 gastrointestinal upset in one participant and a grade 1 elevation in the bilirubin level of another participant during treatment with 800 mg of indinavir, 200 mg of ritonavir, and 40 mg of omeprazole. No participants discontinued the study due to adverse events. The pharmacokinetics for each of the regimens is summarized in Figure 1 and Table 1.
Figure 1.
Mean plasma indinavir concentration versus time profiles in study participants receiving indinavir plus placebo, indinavir plus omeprazole 20 mg, indinavir plus omeprazole 40 mg, and indinavir plus ritonavir and omeprazole 40 mg. Vertical bars indicate standard error of the mean.
Table 1.
Pharmacokinetics of Indinavir for Each Study Regimena
| Treatment | Geometric Mean AUC0-24 (95% CI) (mg · hr/L) | Geometric Mean Cmax (95% CI) (ng/mL) | Geometric Mean tmax (95% CI) (hr) |
|---|---|---|---|
| Indinavir 800 mg plus placebo | 30.0 (21.9–41.1) | 12,600 (9,300–17,200) | 1.1 (0.3–1.9) |
| Indinavir 800 mg plus omeprazole 20 mg | 19.7 (14.6–26.8) | 8,910 (6,530–12,200) | 1.3 (0.5–2.1) |
| Indinavir 800 mg plus omeprazole 40 mg | 16.0 (11.8–21.7) | 7,430 (5,510–10,000) | 1.2 (0.4–2.0) |
| Indinavir 800 mg plus omeprazole 40 mg plus ritonavir 200 mg | 46.6 (34.0–63.8) | 6,590 (4,840–8,970) | 4.1 (3.3–4.9) |
CI = confidence interval.
Pretreatment and coadministration of a 20- or 40-mg dose of omeprazole decreased indinavir exposure after a single-dose administration of 800 mg of indinavir. When compared with placebo, omeprazole 20 mg reduced the geometric mean indinavir AUC from 30.0 mg · hr/L (95% CI, 21.9–41.1 mg · hr/L) to 19.7 mg · hr/L (95% CI, 14.6–26.8 mg · hr/L), but the difference did not reach statistical significance. No statistically significant change was noted in indinavir’s Cmax or tmax after the addition of omeprazole. The minimum plasma concentrations (Cmin) of indinavir were not evaluated.
When compared with placebo, omeprazole 40 mg significantly decreased the geometric mean AUC of indinavir from 30.0 mg · hr/L (95% CI, 21.9–41.1 mg · hr/L) to 16.0 mg · hr/L (95% CI, 11.8–21.7 mg · hr/L). Similar to the 20-mg dose of omeprazole, no statistically significant change was noted in indinavir’s Cmax or tmax. There were no statistically significant differences in AUC, Cmax, and tmax between the 20- and 40-mg omeprazole dosing groups (p ≥ 0.32).
The addition of 200 mg of ritonavir to the 800-mg dose of indinavir after omeprazole 40-mg pretreatment significantly increased the mean indinavir AUC from 16.0 mg · hr/L (95% CI, 11.8–21.7 mg · hr/L) to 46.6 mg · hr/L (95% CI, 34.0–63.8 mg · hr/L). This indinavir AUC was also significantly higher than the indinavir AUC when administered with and after placebo. Although the Cmax of indinavir was not significantly changed with the addition of ritonavir, the tmax was significantly prolonged.
The addition of 200 mg of ritonavir to the 800-mg dose of indinavir after the omeprazole 40-mg pretreatment did not significantly change the geometric mean ± S.E. omeprazole AUC (5.8 ± 0.95 mg · hr/L without ritonavir to 6.1 ± 0.96 mg · hr/L with ritonavir). The mean ± S.E. omeprazole AUC of 40 mg of omeprazole (with or without ritonavir) was significantly higher than the mean ± S.E. omeprazole AUC of 20 mg of omeprazole (6.1 ± 0.96 or 5.8 ± 0.94 mg · hr/L versus 1.9 ± 0.95 mg · hr/L, respectively; both p < 0.0001).
The Cmax ± S.E. of omeprazole was higher with 40 mg of omeprazole plus indinavir versus the 40 mg of omeprazole plus indinavir with the addition of ritonavir (1230 ± 137 ng/mL versus 812 ± 141 ng/mL, p = 0.009). The mean ± S.E. half-life of omeprazole was prolonged with the addition of ritonavir (1.6 ±0.4 hours versus 3.3 ± 0.4 hours, p = 0.0006). There was no significant association between indinavir’s AUC and omeprazole’s AUC.
Discussion
Drug–drug interactions are a challenging aspect of managing patients infected with HIV. The results of this study indicate that, for indinavir’s AUC, a significant drug–drug interaction with PPIs may be overcome by the use of concomitant ritonavir therapy.
Indinavir is poorly soluble in water at physiological pH, with solubility decreasing as pH increases.5 Additionally, indinavir absorption has been shown to be pH-dependent in rats and dogs5 and meal-dependent in healthy volunteers and HIV-infected patients.6 Coadministration of the H2-receptor antagonist cimetidine (600 mg twice daily) and indinavir (a single 400-mg dose) minimally affected indinavir’s AUC.6 As cimetidine is also a CYP3A inhibitor, the influence of the pH changes may have been confounded by the enzyme inhibitory effect.12
In a retrospective analysis of nine HIV-infected patients receiving a combination of indinavir (800 mg three times daily) and omeprazole (20–40 mg daily), four patients had a plasma indinavir AUC below the 95% CI of the average expected population indinavir AUC in patients receiving indinavir alone.13
We chose to perform this pharmacokinetic analysis after a single dose of indinavir rather than under steady-state conditions to decrease attrition, study duration, and medication-related adverse effects in otherwise healthy participants. Although these single-dose healthy-volunteer data may not exactly replicate what would occur in an HIV-infected population, the data are nonetheless compelling and should be considered. Since this study was performed, indinavir use without ritonavir is no longer recommended in an antiretroviral regimen.14 Although indinavir is not frequently used in the developed nations, its availability from generic manufacturers in developing countries still makes indinavir data relevant to HIV treatment worldwide.
To minimize the effect of interpatient variability, a crossover design was used. In the placebo group (indinavir 800 mg alone), indinavir pharmacokinetics varied significantly but was consistent with previously reported pharmacokinetic data.1 Indinavir’s AUC values in our study ranged from 13.7 to 68.7 mg · hr/L and Cmax values ranged from 7,000 to 25,900 ng/mL. However, these values were similar to or slightly higher than the indinavir AUC values reported under steady-state conditions.6
As expected, seven days of omeprazole (40 mg) therapy before indinavir coadministration decreased indinavir exposure. This change can be explained by decreased indinavir solubility and absorption due to an increase in gastric pH. With concurrent administration of 20 mg of omeprazole, the mean indinavir Cmax decreased by 29% and the mean tmax increased by 20%. With 40 mg of omeprazole, the mean indinavir Cmax decreased by 41% and the mean indinavir tmax increased by approximately 10%. With 20 and 40 mg of omeprazole therapy, the mean indinavir AUC0-24 declined by 34% and 47%, respectively. Although there was a trend toward a greater reduction in indinavir’s Cmax and AUC with 40 mg as compared with 20 mg of omeprazole, these differences were not statistically significant. However, there was a statistically significant reduction in the indinavir AUC between 40 mg omeprazole and placebo. Our study was only powered to detect a 50% difference in the AUC between omeprazole 40 mg and placebo.
Coadministration of ritonavir has been shown to eliminate the need for food restrictions (administration without food or with a low-fat meal) in patients receiving indinavir.15,16 However, this is the first study to demonstrate that coadministration of ritonavir with indinavir may offset the pH-dependent decrease in indinavir exposure observed when coadministered in single doses with omeprazole. An increase in indinavir’s AUC0-24 after coadministration of ritonavir is well-known.17 Ritonavir 100 mg coadministered twice daily with 800 mg of indinavir twice daily increases the indinavir Cmax by 1.6-fold and AUC0-24 by 2.7-fold compared with 800 mg of indinavir given alone three times daily. Increasing the ritonavir dose to 200 mg twice daily resulted in a 1.8-fold increase in the indinavir Cmax and a 3.6-fold increase in the AUC0-24.6,17 In this study, 200 mg of ritonavir dosed with indinavir plus 40 mg of omeprazole increased the AUC0-24 of indinavir alone by 2.9-fold compared with indinavir plus 40 mg of omeprazole. This exposure is comparable to historical data of a single dose of indinavir 800 mg administered with 200 mg of ritonavir alone.13 In our study, the administration of indinavir had no effect on the AUC and Cmax of omeprazole. The AUC and Cmax of omeprazole observed in our study were similar to concentrations observed after repeated administration of 40 mg of omeprazole alone.18
In a survey of nonprescription drug use among HIV-infected patients, 56% of patients reported using acid-reducing agents,7 many of which are available without a prescription (e.g., cimetidine, famotidine, nizatidine, ranitidine, omeprazole). Due to the widespread use and availability of these agents, many patients may be subject to interactions between antiretroviral drugs and acid-suppressive therapy.
Fosamprenavir and atazanavir have also demonstrated pH-dependent solubility.4,19,20 In a randomized, open-label, multiple-dose drug interaction study, a 76% decrease in the atazanavir AUC0-24 and a 78% decrease in the atazanavir Cmin were observed when atazanavir 300 mg and ritonavir 100 mg were coadministered with omeprazole 40 mg. Increasing the dose of atazanavir to 400 mg coadministered with 40 mg of omeprazole did not compensate for the decrease in atazanavir exposure.8 Agarwala and colleagues21 evaluated patients receiving atazanavir 300 mg plus ritonavir 100 mg daily coadministered with 40 mg of omeprazole and observed a 61–76% decrease in the AUC of atazanavir. Another open-label crossover study showed that administration of a single 400-mg dose of atazanavir with pretreatment and coadministration of 60 mg of lansoprazole decreased the AUC of atazanavir by 94%.4
In one study, concomitant administration of ranitidine with a single dose of fosamprenavir decreased amprenavir’s AUC and Cmax by 30% and 51%, respectively.19 In another study, coadministration of esomeprazole with fosamprenavir with or without ritonavir had no significant effects on the steady-state amprenavir AUC0-12, Cmax, or Cmin22; this lack of effect may be partially attributable to the administration of fosamprenavir concurrently with esomeprazole at the end of the esomeprazole dosing interval when the effect of gastric pH on fosamprenavir dissolution would have been at a minimum.
Lopinavir, darunavir, and saquinavir pharmacokinetics have also been investigated when combined with nonantacid gastric acid-reducing agents. Concomitant administration of ranitidine, or omeprazole and lopinavir plus ritonavir, or darunavir plus ritonavir has not been shown to significantly alter PI exposure.23-25 Coadministration of saquinavir plus ritonavir with omeprazole has been shown to increase the saquinavir AUC by 82%.26
The data from this study are valuable in demonstrating the impact of combining PPIs with PIs, which have pH-dependent absorption. As previous investigations with indinavir and H2-receptor antagonists did not demonstrate an interaction, these data illustrate the need for drug-interaction studies with both H2-receptor antagonists and PPIs. This concept is important for the development of future PIs with pH-dependent absorption.
Caution should be taken when coadministering indinavir with a PPI. Strong consideration should be given to coadministration with ritonavir, particularly in patients who are suspected of having viral mutations that may cause reduced PI susceptibility.
Conclusion
The AUC of indinavir was substantially decreased in healthy volunteers who received omeprazole 20 or 40 mg daily for seven days before administration of a single 800-mg dose of indinavir. Concomitant administration of ritonavir 200 mg with indinavir in participants receiving omeprazole led to a significant increase in the AUC of indinavir.
Acknowledgments
The assistance of the study volunteers, General Clinical Research Center staff, and investigational drug service is acknowledged.
Supported in part by the UNC Center for AIDS Research, the UNC General Clinical Research Center (RR00046), and the National Institutes of Health.
Biographies
Hiba L. Tappouni, Pharm.D., is Principal Clinical Research Scientist, Psychiatry, GlaxoSmithKline, Research Triangle Park, NC; at the time of writing, she was Drug Development Fellow, Division of Pharmacotherapy and Experimental Therapeutics, School of Pharmacy, University of North Carolina (UNC), Chapel Hill.
John C. Rublein, Pharm.D., is Senior Clinical Science Manager, Abbott Pharmaceuticals, Abbott Park, IL; at the time of writing, he was Clinical Specialist, Infectious Diseases, UNC Hospitals, Chapel Hill.
Brian J. Donovan, Pharm.D., is Clinical Scientific Director, Cubist Pharmaceuticals, Lexington, MA; at the time of writing, he was Specialty Resident, Infectious Diseases Pharmacotherapy, UNC, Chapel Hill.
Stephanie B. Holl owell , Pharm.D., is Clinical Pharmacist, Chippenham Johnston-Willis Medical Center, Richmond, VA.
Hsiao-Chuan Tien, Ph.D., is Senior Statistician, Department of Biostatistics, School of Public Health, UNC, Chapel Hill.
Sherene S. Min, M.D., is Director, Clinical Pharmacology Discovery Medicine, GlaxoSmithKline.
Dickens Theodore, M.D., is Director, Clinical Development, GlaxoSmithKline; at the time of writing, he was Senior Research Physician, GlaxoSmithKline.
Naser L. Rezk, M.S., is Assistant Professor, Division of Pharmacotherapy and Experimental Therapeutics, School of Pharmacy, UNC.
Philip C. Smith , Ph.D., is Associate Professor, Division of Molecular Pharmaceutics, School of Pharmacy, UNC.
Melanie N. Tall man, Pharm.D., is Graduate Student, Division of Molecular Pharmaceutics, School of Pharmacy, UNC.
Ralph H. Raasch , Pharm.D., is Associate Professor, Division of Pharmacotherapy and Experimental Therapeutics, School of Pharmacy, UNC.
Angela D. M. Kash uba, Pharm.D., is Associate Professor, Division of Pharmacotherapy and Experimental Therapeutics, School of Pharmacy, UNC.
Footnotes
Presented at the 43rd Annual Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, IL, 2003.
Vacutainer, Becton, Dickinson and Company, Franklin Lakes, NJ.
BOND ELUT-C18, Varian, Inc., Harbor City, CA.
Series 1100 HPLC System, Agilent Technologies, Wilmington, DE.
Zorbax C-18 analytical column, Agilent Technologies.
Zorbax C-18 guard column, Agilent Technologies.
Omeprazole standard (H153/52), Astra, Sweden.
CAPCELL PAK UG column, Shiseido Co. Ltd., Tokyo, Japan.
HP ChemStation software, Hewlett Packard, Germany.
WinNonlin, V4.01, Pharsight Corporation, Mountainview, CA.
SAS PROC MIXED, version 8, SAS Institute, Inc., Cary, NC.
References
- 1.Acosta EP, Henry K, Baken L, et al. Indinavir concentrations and antiviral effect. Pharmacotherapy. 1999;19:708–12. doi: 10.1592/phco.19.9.708.31544. [DOI] [PubMed] [Google Scholar]
- 2.Flexner C. HIV-protease inhibitors. N Engl J Med. 1998;338:1281–92. doi: 10.1056/NEJM199804303381808. [DOI] [PubMed] [Google Scholar]
- 3.Jackson A, Taylor S, Boffito M. Pharmacokinetics and pharmacodynamics of drug interactions involving HIV-1 protease inhibitors. AIDS Rev. 2004;6:208–17. [PubMed] [Google Scholar]
- 4.Khanlou H, Farthing C. Co-administration of atazanavir with proton-pump inhibitors and H2 blockers. J Acquir Immune Defic Syndr. 2005;39:503–4. doi: 10.1097/01.qai.0000167477.20428.ce. [DOI] [PubMed] [Google Scholar]
- 5.Lin JH, Chen IW, Vastag KJ, et al. pH-dependent oral absorption of L-735,524, a potent HIV protease inhibitor, in rats and dogs. Drug Metab Dispos. 1995;23:730–5. [PubMed] [Google Scholar]
- 6.Crixivan (indinavir sulfate) package insert. Whitehouse Station, NJ: Merck & Co., Inc.; 2005. [Google Scholar]
- 7.Luber AD, Garg V, Gharakhanian S, et al. Survey of medication used by HIV-infected patients that affect gastrointestinal (GI) acidity and potential for negative drug interactions with HAART. Abstract presented at the 7th International Congress on Drug Therapy in HIV Infection; Glasgow, Scotland. 2004 Nov. [Google Scholar]
- 8.Reyataz (atazanavir sulfate) package insert. Princeton, NJ: Bristol-Myers Squibb Company; 2005. [Google Scholar]
- 9.National Institute of Allergy and Infectious Diseases. Division of AIDS toxicity table for grading the severity of adult and pediatric adverse events. [2007 Dec 19];2004 www3.niaid.nih.gov/research/resources/DAIDSClinRsrch/PDF/Safety/DAIDSAEGradingTable.pdf.
- 10.Rezk NL, Tidwell RR, Kashuba AD, et al. High-performance liquid chromatography assay for the quantification of HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2004;805:241–7. doi: 10.1016/j.jchromb.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 11.Kobayashi K, Chiba K, Sohn DR, et al. Simultaneous determination of omeprazole and its metabolites in plasma and urine by reversed-phase high-performance liquid chromatography with an alkaline-resistant polymer-coated C18 column. J Chromatogr. 1992;579:299–305. doi: 10.1016/0378-4347(92)80395-7. [DOI] [PubMed] [Google Scholar]
- 12.Boffito M, Carriero P, Trentini L, et al. Pharmacokinetics of saquinavir co-administered with cimetidine. J Antimicrob Chemother. 2002;50:1081–4. doi: 10.1093/jac/dkf232. [DOI] [PubMed] [Google Scholar]
- 13.Burger DM, Hugen PW, Kroon FP, et al. Pharmacokinetic interaction between the proton pump inhibitor omeprazole and the HIV protease inhibitor indinavir. AIDS. 1998;12:2080–2. doi: 10.1097/00002030-199815000-00025. [DOI] [PubMed] [Google Scholar]
- 14.DHHS panel on antiretroviral guidelines for adults and adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. [2007 Dec 20];2007 AIDSinfo.nih.gov.
- 15.Hsu A. Indinavir can be taken with regular meals when administered with ritonavir. Abstract presented at the 12th World AIDS Conference; Geneva, Switzerland. 1998 Jun. [Google Scholar]
- 16.Saah AJ, Winchell GA, Nessly ML, et al. Pharmacokinetic profile and tolerability of indinavir-ritonavir combinations in healthy volunteers. Antimicrob Agents Chemother. 2001;45:2710–5. doi: 10.1128/AAC.45.10.2710-2715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hugen PW, Burger DM, ter Hofstede HJ, et al. Dose-finding study of a once-daily indinavir/ritonavir regimen. J Acquir Immune Defic Syndr. 2000;25:236–45. doi: 10.1097/00126334-200011010-00005. [DOI] [PubMed] [Google Scholar]
- 18.Hassan-Alin M, Andersson T, Niazi M, et al. A pharmacokinetic study comparing single and repeated oral doses of 20 mg and 40 mg omeprazole and its two optical isomers, S-omeprazole (esomeprazole) and R-omeprazole, in healthy subjects. Eur J Clin Pharmacol. 2005;60:779–84. doi: 10.1007/s00228-004-0841-1. [DOI] [PubMed] [Google Scholar]
- 19.Ford SL, Wire MB, Lou Y, et al. Effect of antacids and ranitidine on the single-dose pharmacokinetics of fosamprenavir. Antimicrob Agents Chemother. 2005;49:467–9. doi: 10.1128/AAC.49.1.467-469.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furfine ES, Baker CT, Hale MR, et al. Preclinical pharmacology and pharmacokinetics of GW433908, a water-soluble prodrug of the human immunodeficiency virus protease inhibitor amprenavir. Antimicrob Agents Chemother. 2004;48:791–8. doi: 10.1128/AAC.48.3.791-798.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agarwala S, Gray K, Wang Y, et al. Pharmacokinetic effect of omeprazole on atazanavir co-administered with ritonavir in healthy subjects. Abstract presented at the 12th Conference on Retroviruses and Opportunistic Infections; Boston, MA. 2005 Feb. [Google Scholar]
- 22.Shelton MJ, Wire MB. Coadministration of esomeprazole with fosamprenavir has no impact on steady-state plasma amprenavir pharmacokinetics ( APV10031). Abstract presented at the 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec, Canada. 2005 Apr; [DOI] [PubMed] [Google Scholar]
- 23.Klein CE, Chiu YL, Cai Y, et al. Lack of effect of acid reducing agents on the pharmacokinetics (PK) of lopinavir/ritonavir (LPV/r) tablet formulation. Abstract presented at the 13th Conference on Retroviruses and Opportunistic Infections; Denver, CO. 2006 Feb. [Google Scholar]
- 24.Sekar V, Hoetelmans R, De Marez T, et al. Pharmacokinetics of TMC114: effect of omeprazole and ranitidine. Abstract presented at the 6th International Workshop on Clinical Pharmacology of HIV Therapy; Quebec, Canada. 2005 Apr. [Google Scholar]
- 25.Sekar V, Hoetelmans H, De Marez T, et al. Pharmacokinetics of TMC114: effect of omeprazole and ranitidine. Abstract presented at the 3rd International AIDS Society Conference on HIV Pathogenesis and Treatment; Rio de Janeiro, Brazil. 2005 Jul. [Google Scholar]
- 26.Winston A, Back D, Fletcher C, et al. Effect of omeprazole on the pharmacokinetics of saquinavir-500 mg formulation with ritonavir in healthy male and female volunteers. AIDS. 2006;20:1401–6. doi: 10.1097/01.aids.0000233573.41597.8a. [DOI] [PubMed] [Google Scholar]