Figure 3. The Generation of pMHC I from N-Terminally Extended Precursors in the ER Requires Enzymatically Active ERAAP.
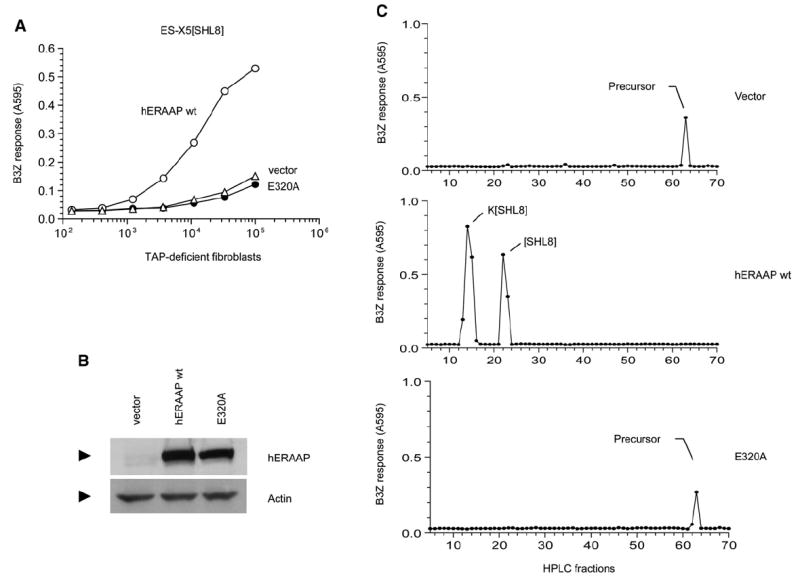
(A) The cDNAs encoding either vector alone, human ERAAP WT, or its single amino acid mutant in the GAMEN motif (E320A) were cotransfected into ERAAP-TAP double-deficient fibroblasts together with the ES-X5[SHL8] construct. After 2 days, expression of SHL8-Kb complex was measured with B3Z T cells.
(B) The hERAAP WT and its E320A mutant are expressed at comparable amounts. The amounts of hERAAP WT, its E320A mutant, or vector alone as a negative control were determined by immunoblotting transfected COS cell lysates with the hERAAP antibody.
(C) The conversion of the N-terminally extended precursor to the final SHL8 or K[SHL8] peptides presented by Kb or Db MHC occurs only in the presence of hERAAP WT. The peptide extracts from ERAAP-TAP double-deficient fibroblasts transfected with ES-X5[SHL8] and either vector alone, hERAAP WT, or its E320A mutant were fractionated by RP-HPLC. The SHL8-containing, N-terminally extended intermediates were detected after trypsin treatment of each fraction and incubating them with B3Z T cells in the presence of the appropriate APC. The peaks were identified, where known, by comparison with synthetic peptides run under identical conditions. Data are representative of at least two independent experiments.
