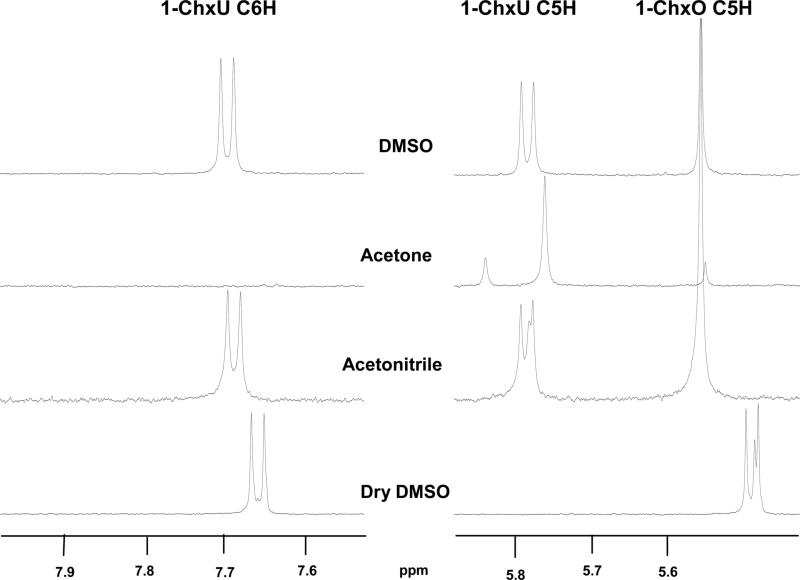
Figure 4.
1H NMR spectra of the C6 and C5 protons of 1-ChxU arising from the decarboxylation of 1-ChxO conducted in three solvents. The top three samples were diluted in D2O, while the fourth was diluted in DMSO-d6.When traces of H2O were present in these reactions, two doublets (J = 7.9 Hz) were produced when the C6 carbanion extracted a proton from water. In acetone-d6, the C6-carbanion extracted a deuteron from the solvent, leaving only the C5 proton resonance as a singlet. Acetonitrile-d3 and “dry” DMSO showed evidence of both reactions. In DMSO-d6, the resonances were shifted upfield from their positions in D2O.