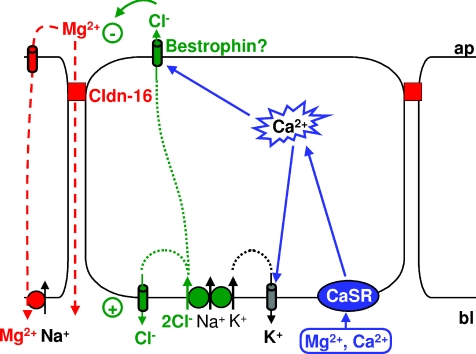
Figure 9. Model of components involved in the generation of the divalent cation-induced Cl− current in claudin-16-transfected MDCK-C7 cells.
Application of divalent cations to the basolateral side of claudin-16-expressing MDCK-C7 cell layers activates the Ca2+-sensing receptor, CaSR. This causes release of Ca2+ from intracellular stores and thus an increase in [Ca2+]i. Intracellular Ca2+ activates the apical Ca2+-sensitive Cl− channel bestrophin, which, in claudin-16-expressing cells, is recruited to the apical membrane and to the tight junction, i.e. close to the release sites of Ca2+ from intracellular stores. Cl− enters the cells through basolateral Na+-dependent, K+-dependent or independent symport mechanisms and, under resting conditions, probably leaves the cells through basolateral, DIDS-sensitive Cl− channels. When, upon CaSR stimulation, apical Cl− channels open, Cl− is able to leave the cells on the apical side, thus carrying the observed current. K+ entering the cells together with Cl− on the basolateral side can leave the cells on the basolateral side through Ca2+-sensitive K+ channels. This helps to further enhance the observed divalent cation-induced current. This current changes the transepithelial potential and thus the driving force for both para- and transcellular transport of Mg2+ (and Ca2+).