Fig. 2.
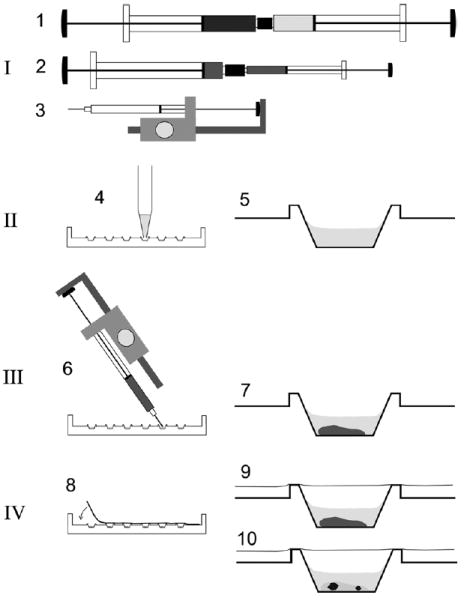
Schematic procedure for the rapid preparation of micro crystallization experiments using the cubic phase method. Crystallization setups are prepared using syringes in combination with a semi-automatic dispenser-driven micro syringe and Terasaki plates. The procedure involves four basic steps: [I] Preparation of the proper lipidic cubic phase using two 250 μl gas-tight syringes coupled together as described [2] (1). Typically, the cubic phase consists of ca. 60% monoolein, ca. 40% water and protein—i.e., bacteriorhodopsin—at a concentration of ca. 3.5 mg/ml in 20 mM Sørensen phosphate buffer, pH 5.6. For dispensing into microwells, the material is transferred into a microsyringe with 10 μl total volume either directly into the syringe barrel or via an appropriate adapter (2). This microsyringe is assembled into a semi-automatic dispenser (3) which allows dispensation in 50 steps of ca. 0.2 μl each. [II] Prior to dispensation of the lipid material, six microwells in one lane of a 72-well Terasaki plate are filled with 1 μl of the precipitant solutions (4) and dried optionally at 60 °C for 30 min. The latter resembles crystallization of bacteriorhodopsin using dry salt as the precipitation agent as described previously [9,11,23]. A single well is shown on the right (5). [III] Ca. 0.2 μl of the preformed lipidic material is added to each microwell by positioning the shortened syringe needle at an angle over the well bottom and by triggering the semi-automatic dispenser (6). The average weight of a single shot of 60% (w/v) monoolein containing cubic phase using the dispenser was determined to be 222 ± 10.3 μg. The cubic phase is stable in an excess of an overlaying liquid (7). [IV] The microwells are sealed with a clear transparent tape (8) and the plates may be stored (9) at different temperatures. Inspection of crystals (10) with a microscope can be carried out without disturbance from both sides simply by flipping the entire plate.
