Figure 3. Interdisciplinary development of nanomedicines for HIV-1 encephalitis and other human diseases.
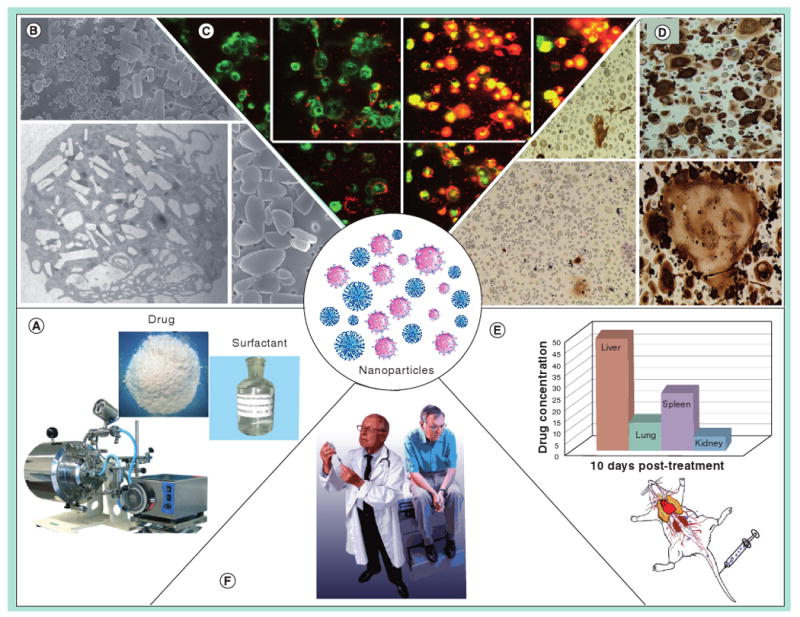
Modalities that are site specific hold considerable promise for human use and their development is illustrated herein. (A) Neuroprotective, anti-inflammatory, anti-apoptotic or antimicrobial agents are packaged into nanoparticles (NPs) with surfactant and protein coats that target circulating immunocytes or diseased regions within the CNS. (B) The NPs are developed in size, shape, charge and coating to facilitate their uptake into circulating monocytes. (C) The cells circulating in the body will scavenge the particles into cytoplasmic vesicles as a delivery vehicle. Laboratory tests can measure uptake and release of fluorescently tagged drug-laden NPs from monocytes and monocyte-derived macrophages (D). In the case of HIV-1 infections, for example, the drug-laden NPs not housed within monocyte-derived macrophages would release antiretroviral drugs leading to inhibition of viral replication, measured by the absence of multinucleated giant cells and HIV-1 p24 protein expression. (E) Such formulations can then be tested in animals demonstrating sustained drug levels for an extended period. (F) Final therapeutic use in humans will be dependent upon toxicity measures, pharmacokinetic responses and untoward side effects.
