Figure 4.
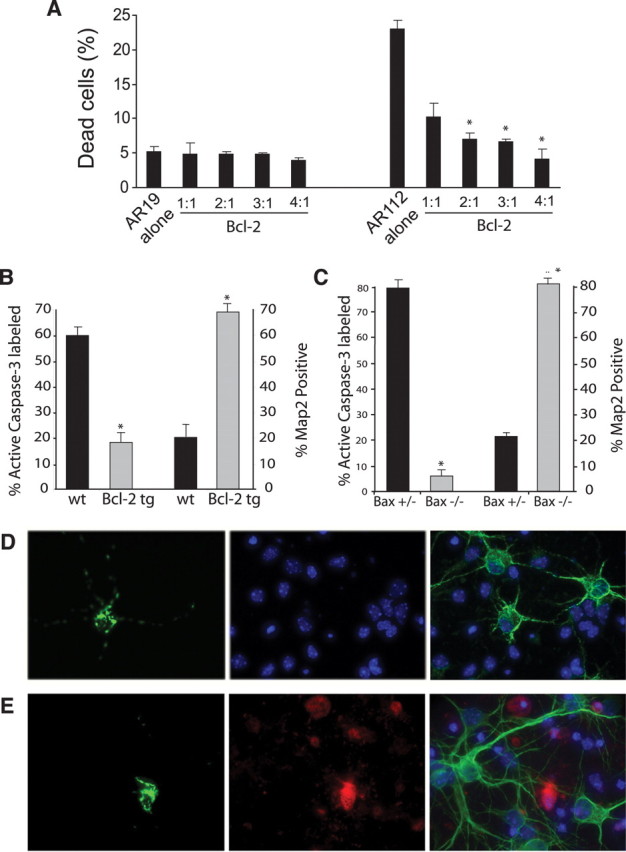
AR112 truncation fragment expression activates apoptosis via the intrinsic pathway. A, Bcl-2 protects MN-1 cells against AR112-induced cell death (*p < 0.01; Student's t test). B, Primary cortical neurons derived from Bcl-2 transgenic mice are strongly protected against AR112 neurotoxicity (*p < 0.01; ANOVA). C, Primary cortical neurons derived from Bax null mice are fully protected from AR112 neurotoxicity (*p < 0.01; ANOVA). D, E, Here we see representative images of AR-transfected neurons using four-channel imaging: DAPI (blue), GFP (green, left), Cy3-labeled active caspase-3 (red), and Cy5-labeled MAP2 (green, right). D, Transfection of Nt-AR112-GFP into Bax null neurons does not cause toxicity. Left, We see a neuron expressing AR112. At 24 h after transfection, no active caspase-3 (red) is detected in the AR112-transfected neuron (middle). Cy5 (green) labeling of MAP2 reveals normal, healthy neurites in this AR112-expressing neuron (right). E, Transfection of Nt-AR112-GFP into Bax+/− neurons does produce neurotoxicity. Left, We see a neuron expressing AR112. At 24 h after transfection, immunostaining for active caspase-3 (red) strongly labels this AR112-expressing neuron (middle). While numerous nontransfected cortical neurons exhibit healthy MAP2-positive processes (Cy5, green), the AR112-expressing cortical neuron displays no MAP2 staining (right). In all cases, nuclear condensation indicative of neuron cell death corresponded to caspase-3 activation in these experiments (data not shown).
