Abstract
The ability to detect and identify targets in cluttered scenes is a critical skill for survival and interactions. To solve this challenge the brain has optimized mechanisms for capitalizing on frequently occurring regularities in the environment. Although evolution and development have been suggested to shape the brain's architecture in a manner that resembles these natural statistics, we provide novel evidence that short-term experience in adulthood may modify the brain's functional organization to support integration of signals atypical of shape contours in natural scenes. Although collinearity is a prevalent principle for perceptual integration in natural scenes, we show that observers learn to exploit other image regularities (i.e., orthogonal alignments of segments at an angle to the contour path) that typically signify discontinuities. Combining behavioral and functional MRI measurements, we demonstrate that this flexible learning is mediated by changes in the neural representations of behaviorally relevant image regularities primarily in dorsal visual areas. These changes in neural sensitivity are in line with changes in perceptual sensitivity for the detection of orthogonal contours and are evident only in observers that show significant performance improvement. In contrast, changes in the activation extent in frontoparietal regions are evident independent of performance changes, may support the detection of salient regions, and modulate perceptual integration in occipitotemporal areas in a top-down manner. Thus experience at shorter timescales in adulthood supports the adaptive functional optimization of visual circuits for flexible interpretation of natural scenes.
INTRODUCTION
The ability to detect and identify targets in cluttered scenes is a skill critical for many of our interactions in the complex environments we inhabit: identifying predators and prey in natural scenes, recognizing friends in the crowd, detecting objects in medical or security images. It is therefore conceivable that the visual system has evolved to capitalize on statistical regularities in the environment for extracting shape information from the noisy sensory input. Supporting evidence comes from behavioral and computational work showing that observers are better at detecting collinear edges (i.e., edges aligned along a path) (Dakin and Hess 1997; Field et al. 1993; Hess and Field 1999) that co-occur frequently and form contours in natural images (Geisler 2008; Geisler et al. 2001; Sigman et al. 2001). In contrast, edges oriented at an angle with respect to a path (e.g., orthogonal or acute alignments) co-occur less frequently in natural scene contours and have been reported to be more difficult to detect (Bex et al. 2001; Field et al. 1993; Ledgeway et al. 2005).
These findings suggest an instructive role of experience in the encoding of statistical regularities by the visual system. Although evolutionary and developmental influences have been hypothesized to contribute to the long-term optimization of the visual system for typical natural contour statistics (e.g., collinearity; Gilbert et al. 2001; Simoncelli and Olshausen 2001), our recent behavioral studies show that short-term experience in adulthood may modify the behavioral relevance (i.e., utility) of atypical contour statistics for the interpretation of natural scenes (Schwarzkopf and Kourtzi 2008). In particular, observers learn to use discontinuities typically associated with surface boundaries (orthogonal alignments) for contour linking and detection. However, the experience-dependent plasticity mechanisms in the human brain that mediate this flexible exploitation of natural statistics remain largely unknown.
Here we combine psychophysical and functional magnetic resonance imaging (fMRI) measurements to investigate the neural basis of learning image regularities. We chose stimuli that violate the grouping principle of collinearity. That is, we investigated learning of orthogonal alignments that are more likely to indicate discontinuities (i.e., texture boundaries) and serve as a cue for surface segmentation rather than contour integration (Elder and Goldberg 2002; Geisler 2008; Geisler et al. 2001; Kruger and Worgotter 2002; Sigman et al. 2001). We tested the ability of observers to detect contours (Fig. 1A) embedded in noise (i.e., background of randomly oriented Gabor elements) when the Gabor elements defining the contours were 1) aligned along the contour path (collinear contours), 2) oriented orthogonally to the contour path (orthogonal contours), or 3) at an angle of 30° to the path (acute contours). We compared detection performance and fMRI activations for these contour types before and after training on orthogonal contours (2,400–3,600 trials, over four to six daily sessions).
FIG. 1.
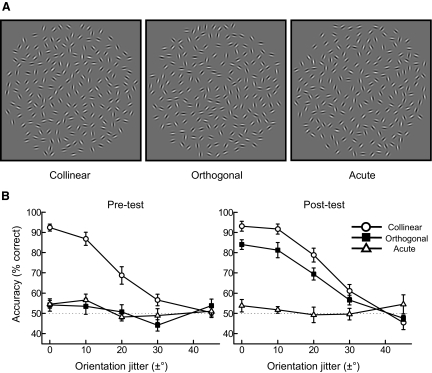
Stimuli and behavioral data. A: examples of stimulus conditions: collinear contours in which elements are aligned along the contour path, orthogonal contours in which elements are oriented at 90° to the path, and acute contours in which elements are oriented at 30° to the path. Contours at different global orientations are shown for each condition. B: psychometric curves (average across observers, n = 8) for contour detection performance (percentage correct) plotted as a function of local orientation jitter before training (pretest) and after training (posttest). Circles: collinear contours; squares: orthogonal contours; triangles: acute contours. Error bars denote ±SE across observers. Psychometric curves for individual participants are shown in Supplemental Fig. S9.
Consistent with long-term optimization for typical scene statistics, observers were better at detecting collinear than orthogonal or acute contours. However, training to detect orthogonal contours resulted in improved detection performance similar to that for the detection of collinear contours that did not transfer to acute contours. These findings replicated our previous behavioral results (Schwarzkopf and Kourtzi 2008), providing evidence that short-term experience enhances the observers' sensitivity to camouflaged targets by assigning new utility to image regularities. Importantly, our fMRI results provide evidence for a strong link between learning of image regularities and changes in neural representations primarily in dorsal visual areas. In particular, training changed the information content and distribution of the responsive voxels in these areas, suggesting changes in neural sensitivity for features that are relevant for the detection of orthogonal contours. In contrast, gain modulation of cortical activity that may relate to behavioral salience and stimulus familiarity was observed in an extensive occipitotemporal and frontoparietal network. Interestingly, changes in the neural representation of orthogonal contours were observed for learners rather than “weak” learners who did not improve significantly with training and showed gain modulation of activations in occipitotemporal and frontoparietal areas. Our findings provide novel evidence that the changes in neural sensitivity primarily in dorsal visual areas relate to enhanced behavioral sensitivity after training and may support flexible learning of image regularities for the detection of camouflaged targets.
METHODS
Participants
Eleven observers from the University of Birmingham participated in the first experiment (mean age: 24 yr, range: 20–34) and five observers participated in the second experiment (mean age: 26 yr, range: 23–32). All observers had normal or corrected-to-normal vision; gave written consent; were naïve to the hypotheses, stimuli, and experimental procedure; and were paid for their participation. The study was approved by the local ethics committee.
Stimuli
Detailed information on stimulus generation was described in a previous study using the same stimuli (Schwarzkopf and Kourtzi 2008). Here, we describe in brief the relevant stimulus parameters. Stimuli were Gabor fields (8° of visual angle in diameter) that consisted of 200 elements (wavelength: 0.2°; SD: 0.3°) presented within a circular aperture. Each stimulus comprised five parallel contour paths, embedded within a background of randomly oriented distractors placed on a jittered 16 × 16 grid (cell size = 0.5°). Gabor elements comprising the target contours fell on straight, invisible backbone paths subtending ≤5.9° of visual angle (nine Gabor elements). The global orientation of the contours varied between 15° and 165° (in increments of 30°). The distance between contours was randomized within a range of 1.6°–1.9° and the position of the contours was jittered along the global orientation axis across trials. This ensured that all five contours were embedded within the field of random background elements and learning of local configurations between target and background elements was not likely across trials. This stimulus design together with the brief stimulus presentation and central fixation instructions to the observers ensured that detection performance could not be attributed simply to the detection of contours near the edge of the stimulus aperture. The interelement spacing was randomly jittered (mean spacing = 0.7°) to prevent density cues, but elements were kept at a minimum spacing of 0.4°. Three stimulus conditions (see Fig. 1A for example stimuli) were defined by the local orientation alignment with respect to the global orientation axis of the contour. That is, individual elements could either be aligned along the path (collinear contours), perpendicular (orthogonal contours), or at an angle of 30° (acute contours) to the contour path.
For the psychophysics training and test sessions, we parametrically varied the strength of the contour by adding a local orientation jitter (±0°, ±10°, ±20°, ±30°, or ±45°) to each element that misaligned it from the mean orientation. Each stimulus configuration (contour and background) was presented only once across trials to avoid learning of local relations between contour and background elements. In addition, we generated random stimuli that were created by shuffling the local orientations of all the elements in the field. Thus for every stimulus in each condition (collinear, orthogonal, acute) we generated a random stimulus. As a result all contour types and random stimuli were matched for the local position and the orientation distributions of the Gabor elements. This manipulation makes it unlikely that the observers' detection performance could be driven by computing the dominant stimulus orientation in the stimulus in that this would result in similar performance for all contour types before and after training.
The same stimuli were used for the fMRI experiments. In the first fMRI experiment, we tested collinear (0° jitter), collinear-jittered (±45° jitter), orthogonal (0° jitter), orthogonal-jittered (±45° jitter), acute (0° jitter), and acute-jittered (±45° jitter). In the second fMRI experiment, we tested stimuli containing collinear and orthogonal contours presented at two orientations: global contour orientations near the left (135 ± 5°) or the right diagonal (45 ± 5°). That is, we tested four contour conditions: collinear near 45°, collinear near 135°, orthogonal near 45°, orthogonal near 135°. In addition we included two random contour conditions. The second random condition was generated by rotating 40 elements (the average number of elements that would appear in the target contours) in the first random condition by 90°. This ensured that the two random conditions contained on average the same orientation difference as the orientation difference between collinear and orthogonal contours.
Design and procedure
All observers participated in six psychophysics sessions (minimum) and two fMRI sessions that were conducted on different days. On the first day observers participated in a psychophysical test (pretest) to evaluate their ability to detect collinear, orthogonal, and acute contours in noise. In the following session, observers were scanned to measure fMRI responses to the three contour conditions before training (prescan). On subsequent days observers were trained with feedback on orthogonal contours in the psychophysics lab. Training lasted for at least four sessions. Five observers who had not achieved 80% correct detection performance for stimuli with 0° local orientation jitter on the fourth sessions were trained further, but never longer than six sessions (three observers were trained for five sessions, two observers were trained for six sessions). In the final psychophysics session observers were tested after training on the three contour conditions without feedback (posttest). The second scanning session measured the observers' fMRI responses to the stimuli after training (postscan).
PSYCHOPHYSICAL TEST SESSIONS.
All observers completed two psychophysical test sessions: one prior to training (pretest) and one after training (posttest). Before the first test session, observers completed a brief familiarization phase (72–144 trials) during which they were presented with stimuli from all conditions for a longer duration and they received auditory feedback on incorrect responses. The posttest session was always conducted on the day following the final training session. In all sessions, observers viewed the stimuli on a computer screen (resolution 1,280 × 1,024) at a distance of 65 cm in a darkened room. The task was a two-interval forced-choice (2IFC) detection task. On each trial observers were asked to fixate a small black cross (0.18°) in the center of a screen on a uniform gray blank screen. After 400 ms, two Gabor field stimuli were presented in intervals of 300 ms, separated by a 1,000-ms interstimulus interval during which only the fixation cross was presented. Subsequently, the fixation cross disappeared and observers were asked to make a behavioral response indicating which of the two intervals contained the contours by clicking a mouse button. The intertrial interval was 500 ms. Observers were instructed to respond as accurately and quickly as possible and did not receive feedback on their judgments. Observers completed 540 trials per session (i.e., 36 trials per level of orientation jitter per stimulus condition). In the first experiment, the three stimulus conditions (collinear, orthogonal, and acute) were blocked to prevent differences in criterion that could confound the results. In the second experiment, stimulus conditions were randomly interleaved to test for learning effects when the stimulus condition was unpredictable (Kuai et al. 2005).
PSYCHOPHYSICAL TRAINING SESSIONS.
The task and procedure during the training sessions were the same as those during the pretest and posttest sessions. However, during training observers were presented with orthogonal contours only and received auditory feedback (a short beep of 600 Hz and 150-ms duration) on incorrect responses. Training on the 2IFC detection task was carried out for four to six sessions (600 trials per session), usually on consecutive days.
FMRI SESSIONS.
Observers were scanned twice: once before training (after the pretest psychophysical session) and once after training (after the posttest psychophysical session). Each scanning session comprised nine runs, each of which lasted 5 min 20 s. A run comprised twenty 16-s-long blocks: 18 stimulus blocks and 2 fixation blocks in the beginning and the end of the run during which only the fixation cross was presented. Each of the six stimulus conditions was presented three times in a counterbalanced order across runs. Twenty stimuli were presented per block. Each stimulus was presented for 250 ms each followed by 550 ms blank. Observers performed a target-detection task that required them to attend to the stimuli similarly across all conditions. That is, observers were instructed to detect collinear stimuli at cardinal orientations (0° and 90°) with reduced interelement spacing (0.33°) that enhanced the visibility of these target contours. Two target stimuli were randomly interspersed within each block, with the constraint that two targets could never appear in consecutive trials.
Imaging: data acquisition
The experiments were conducted at the Birmingham University Imaging Centre using a 3-Tesla Philips Achieva MRI scanner. T2*-weighted functional and T1-weighted anatomical (1-mm isotropic resolution) data were collected with an eight-channel SENSE head coil. EPI data (gradient echo-pulse sequences) were acquired from 32 slices (whole brain coverage: repetition time, 2,000 ms; time to echo, 35 ms; 2.5 × 2.5 × 3-mm resolution).
fMRI data analysis
DATA PREPROCESSING.
Neuroimaging data were processed using Brain Voyager QX (Brain Innovation, Maastricht, The Netherlands). Anatomical data were used for three-dimensional (3D) cortex reconstruction, inflation, and flattening. Preprocessing of the functional data involved slice-scan time correction, 3D head movement correction, temporal high-pass filtering (three cycles), and removal of linear trends. No spatial smoothing was performed on the functional data used for the multivariate analysis. The functional images were aligned to anatomical data and the complete data transformed into Talairach space. For each participant, the functional imaging data between the two sessions (before and after training) were coaligned, registering all volumes of each subject to the first functional volume. This procedure ensured a cautious registration across sessions. To avoid confounds from any remaining registration errors we compared fMRI signals between stimulus conditions (contour types) within each session rather than across sessions.
UNIVARIATE FMRI ANALYSIS.
We used a general linear model (GLM), with predictors for each of the stimulus conditions and each of the six degrees of freedom of the 3D motion correction. For each session (before and after training) we computed contrasts for all the possible combinations of stimulus conditions. That is, we contrasted each contour type condition against the corresponding jittered condition and the other contour conditions. Comparison of these contrasts across sessions (i.e., before and after training) allowed us to identify cortical regions that showed an interaction between stimulus condition and session, suggesting that differential fMRI responses across stimulus conditions were modulated by learning.
Further, we identified cortical areas that showed significantly higher responses for the collinear than for the collinear-jittered stimuli (Talairach coordinates; Supplemental Table S1)1 using random-effects (RFX) GLM across all observers (P < 0.05, cluster-size threshold corrected, 80 mm2). We then localized these collinear-responsive regions for each individual observer using data from the first scanning session (P < 0.05, cluster-size threshold correction). In addition, for each observer we identified retinotopic visual areas using standard retinotopic mapping procedures (DeYoe et al. 1996; Engel et al. 1994; Sereno et al. 1995) (see Supplemental material).
MULTIVOXEL PATTERN ANALYSIS.
For each region of interest (ROI; collinear-responsive regions, retinotopic areas), we sorted the voxels according to their response (t-statistic) to all stimulus conditions compared with fixation baseline across all experimental runs. We selected the same number of voxels across ROIs and observers by restricting the pattern size to the minimum number of voxels across all ROIs and subjects with a P value <0.05 for the “all conditions versus fixation” contrast. This procedure resulted in the selection of 152 voxels per ROI in the first fMRI experiment (with the exception of <50 voxels in temporal and frontoparietal regions for one participant that were not included in further analyses) and 120 voxels in the second experiment, comparable to the dimensionality used in previous studies (Haynes and Rees 2005; Kamitani and Tong 2005). We normalized (z-score) each voxel time course separately for each experimental run to minimize baseline differences across runs. The data vectors for the multivariate analysis were generated by shifting the fMRI time series by 4 s to account for the delay of the hemodynamic response and then averaging all time series data points of one experimental block. We used a Support Vector Machine (SVMlight toolbox; Supplemental material) for pairwise classification of contour conditions: collinear versus orthogonal, orthogonal versus acute, collinear versus acute. We performed an eightfold cross-validation, leaving one run out (test sample); that is, we used data from eight runs as training patterns (48 patterns: 6 patterns per run, 3 per contour type) and data from the remaining run as test patterns (6 patterns). For each subject we averaged the accuracy (number of correctly assigned test patterns/total number of assignments) across cross-validations. Statistical significance across subjects was evaluated using repeated-measures ANOVA.
RESULTS
Psychophysical results
We tested the ability of observers (n = 8) to detect collinear, orthogonal, and acute contours in a two-interval forced-choice task (i.e., observers judged which of two sequentially presented stimuli contained a global contour) before (pretest session) and after (posttest session) training on orthogonal contours (2,400–3,600 trials, over four to six daily sessions). Before training observers were more sensitive to collinear than orthogonal or acute alignments, consistent with previous studies (Bex et al. 2001; Field et al. 1993; Hess and Field 1999; Hess et al. 2000; Ledgeway et al. 2005), providing evidence for the strength of collinearity as a cue for contour integration in natural scenes. However, training enhanced the ability of observers to detect orthogonal contours (Fig. 1B), consistent with our previous findings (Schwarzkopf and Kourtzi 2008). We quantified improvement in the contour-detection task during training by calculating the accuracy (percentage correct) at zero local orientation jitter before and after training. Accuracy for orthogonal contour detection increased across training sessions (Supplemental Fig. S1) and was significantly higher after than that before training. In particular, a repeated-measures ANOVA showed a significant [F(2,14) = 34.5, P < 0.001] interaction between stimulus (collinear, orthogonal, acute) and session (pretest, posttest) and significant (contrast analysis) performance improvement for orthogonal contours after training [F(1,7) = 46.4, P < 0.001]. Further, a small but significant advantage in performance for collinear over orthogonal contours was observed after training [t(7) = 3.8, P < 0.01]. However, no significant differences were observed in detection performance for acute contours before and after training [F(1,7) < 1, P = 0.73], suggesting that training resulted in learning specific to the trained contour alignment (orthogonal contours) rather than general task improvement.
fMRI results
To investigate experience-dependent brain changes related to contour detection, we measured fMRI activations to collinear, orthogonal, and acute contours in two fMRI sessions, one before and one after the observers were trained on the orthogonal contour detection. Observers were presented with the three contour types (intact stimuli) and their jittered versions and performed a target-detection task (i.e., the target stimulus was a collinear contour at cardinal orientations). Similar performance across conditions in this task indicated that observers maintained attention similarly across conditions and sessions (see Supplemental data).
Training-dependent changes in activation magnitude: intact versus jittered contours
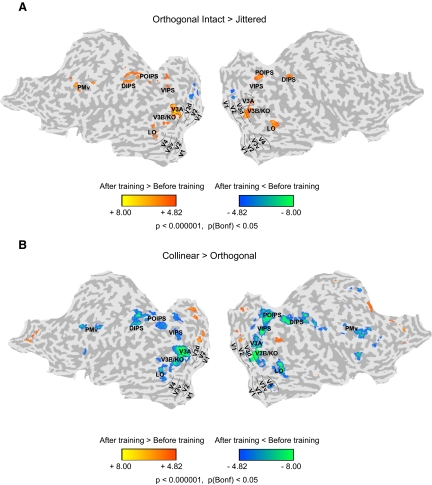
Consistent with behavioral improvement in the detection of orthogonal contours, we observed increased fMRI activations for orthogonal contours after than before training (Fig. 2A). In particular, after training we observed significantly higher activations (P < 0.05, Bonferroni corrected) for intact than for jittered orthogonal contour stimuli in dorsal (intermediate dorsal regions V3A and V3B/kinetic occipital [KO]) extrastriate areas, temporal (lateral occipital sulcus [LO]), intraparietal (ventral, parietooccipital, and dorsal intraparietal sulci [VIPS, POIPS, and DIPS]) cortex, and premotor areas (ventral portion [PMv]). Interestingly, the activation patterns observed for orthogonal contours after training were similar to those observed for collinear intact compared with jittered contours (Supplemental Fig. S2). Consistent with the behavioral results showing lack of learning transfer for acute contours, only weak differences in fMRI activation patterns were observed across sessions (before vs. after training) for acute intact versus jittered contours (Supplemental Fig. S3).
FIG. 2.
Univariate functional magnetic resonance imaging (fMRI) analysis: orthogonal intact vs. jittered contours. A: group general linear model (GLM) maps showing significant differences between sessions (before and after training) for the differential fMRI response to orthogonal intact vs. jittered contours. Data are presented on a flattened reconstruction of 2 cortical hemispheres. Positive t-values indicate that the difference in responses to intact vs. jittered orthogonal contours after training was larger than that before training (P < 0.05, Bonferroni corrected). Dotted lines indicate the borders between retinotopic visual areas. fMRI data from individual participants are shown in Supplemental Fig. S9. B: group GLM maps showing significant differences between sessions (before and after training) for the differential fMRI response to collinear vs. orthogonal contours. Data are presented on a flattened reconstruction of 2 cortical hemispheres. Negative t-values indicate that the difference in responses to collinear vs. orthogonal contours after training was lower than that before training (P < 0.05, Bonferroni corrected). Dotted lines indicate the borders between retinotopic visual areas.
To further quantify these training-dependent changes, we compared fMRI responses before and after training in independently defined regions of interest (Supplemental Fig. S4). In particular, for each observer we identified collinear-responsive regions (i.e., regions that showed stronger fMRI responses for collinear intact than jittered stimuli) and retinotopic regions and calculated percentage signal change from fixation baseline for collinear and orthogonal contours before and after training. A repeated-measures ANOVA showed stronger activations after than before training for orthogonal contours in higher occipitotemporal and parietal regions rather than early visual areas, as supported by a significant [F(28,196) = 2.24, P < 0.01] interaction between stimulus condition (collinear, orthogonal), session (before, after training), and region of interest. In particular, training resulted in increased fMRI responses for orthogonal contours after training in dorsal visual (V3d, V3A, V3B), temporal (ventral occipitotemporal region [VOT], LO, posterior fusiform gyrus[pFs]), and parietal (VIPS, POIPS, DIPS) regions, whereas decreased fMRI responses for collinear contours in temporal and frontoparietal regions. This was supported by significant interactions between stimulus condition (collinear, orthogonal) and session (before and after training) in dorsal visual [F(2,14) = 2.08, P < 0.05], temporal [F(2,14) = 8.92, P < 0.01], and frontoparietal [F(2,14) = 22.33, P < 0.001] regions, but not ventral [V3v, V4: F(2,14) < 1, P = 0.40] or early [V1, V2: F(2,14) < 1, P = 0.98] visual areas.
Taken together, these results demonstrate a link between training-dependent behavioral improvement and fMRI activations. In particular, enhanced performance in the detection of orthogonal contours after training was associated with increased cortical activations for orthogonal contours compared with jittered stimuli in dorsal visual, temporal, and frontoparietal regions, consistent with the role of these areas in perceptual grouping and learning (Dolan et al. 1997; Fink et al. 1996; Ostwald et al. 2008). Interestingly, training on orthogonal contours resulted in a smaller but significant behavioral improvement for collinear contours that was associated with decreased activations for collinear contours in temporal and frontoparietal regions. These results are consistent with previous imaging studies showing decreased fMRI activations for training of salient stimuli for which performance has saturated (Kourtzi et al. 2005; Yotsumoto et al. 2008) that may relate to a more efficient stimulus representation by smaller neural ensembles. Finally, no significant learning-related changes were observed for acute contours, consistent with the lack of learning transfer for these contour stimuli for the training period used in this study (see also Schwarzkopf and Kourtzi 2008).
Training-dependent changes in activation magnitude: comparing contour types
We further investigated differences in the fMRI activation patterns between different contour types (collinear vs. orthogonal, orthogonal vs. acute, collinear vs. acute) before and after training. The main comparison of interest is between collinear and orthogonal contours because the observers' performance for acute contours remained at chance after training. According to the behavioral results, we reasoned that differences in fMRI responses between collinear and orthogonal contours would decrease as detection performance improved for orthogonal contours after training. Consistent with this prediction, GLM analysis (P < 0.05, Bonferroni corrected) showed that activations for collinear versus orthogonal contours decreased significantly after training in dorsal visual, temporal, and frontoparietal regions (Fig. 2B). Comparing activations for orthogonal (Supplemental Fig. S5A) or collinear (Supplemental Fig. S6A) contours to acute contours showed similar results as when comparing activations for intact to jittered contours. This is consistent with the poor detection performance for acute contours observed after training.
Training-dependent changes in information content: comparing contour types
The univariate (GLM, percentage signal change comparisons) analyses described earlier showed that behavioral improvement after training is associated with changes in the magnitude of the blood ovygenation level–dependent response in higher occipitotemporal and frontoparietal regions. These changes were expressed as increases in activation for orthogonal contours but decreases for collinear contours. To gain further insight into the training-dependent changes in the information content of the underlying neural populations, we used advanced multivoxel pattern analysis (MVPA) methods for fMRI data analysis (Cox and Savoy 2003; Haynes and Rees 2006; Norman et al. 2006). These methods take advantage of information across voxel patterns and have been shown to be more sensitive than conventional brain imaging approaches that average across neural populations with differential selectivity within a given voxel. We exploit the sensitivity of these methods to discern differences in the processing of different contour types (collinear vs. orthogonal, orthogonal vs. acute, collinear vs. acute) before and after training. In particular, for each session (pre-, posttraining) we trained a linear support vector machine (SVM) to discriminate between fMRI activation patterns associated with the different contour types. Using a leave-one-run-out cross-validation procedure, we tested the accuracy of this classifier in predicting the contour type presented to the observers based on fMRI activation patterns from an independent data set. We compared the average (across cross-validations and observers) classification accuracy (at voxel pattern size = 152 voxels) before and after training for each ROI.
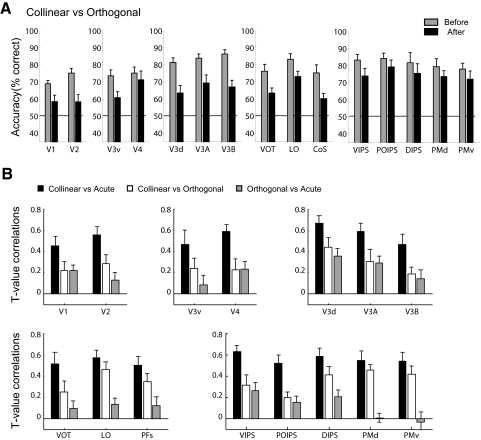
Figure 3A shows the results of the MVPA for the main comparison of interest: collinear versus orthogonal contours. Consistent with higher detection performance for collinear than orthogonal contours before training, classification accuracy for collinear versus orthogonal contours was significantly higher before than that after training [F(1,7) = 12.04, P = 0.01]. However, a significant [F(14,98) = 2.73, P < 0.01] interaction between session (before, after training) and ROI showed that classification accuracy for collinear versus orthogonal contours significantly decreased after training in early retinotopic [F(1,7) = 18.82, P < 0.01], dorsal extrastriate [F(1,7) = 20.52, P < 0.01], and temporal areas [F(1,6) = 10.13, P < 0.05], but not in frontoparietal areas [F(1,6) = 2.79, P = 0.15] and only marginally in ventral areas [F(1,7) = 3.79, P = 0.09].
FIG. 3.
Multivariate fMRI analysis: comparison of contour types. A: multivoxel pattern analysis (MVPA) accuracy (percentage correct) per region of interest (ROI) (voxel-pattern size of 152 voxels) for classification of collinear vs. orthogonal contours. Gray bars: before training; black bars: after training. Error bars denote SE across observers. B: mean correlation coefficients (R) between the t-test values for each voxel expressing the difference in fMRI responses between contour types before and after training. Coefficients are plotted across ROIs for each of 3 contrasts between contour types: collinear vs. acute (black), collinear vs. orthogonal (white), orthogonal vs. acute (grey). Error bars denote ±SE across observers.
To control for the possibility that the classification accuracies we observed were not simply due to low-level differences between the contour types (i.e., position, orientation of local elements), we conducted the same MVPA on the jittered (collinear vs. orthogonal) contours that differed at the local orientation of the contour elements by the same magnitude (90°) as that of the intact contours. Classification accuracy for collinear versus orthogonal jittered stimuli was not significantly different from chance before or after training across ROIs (Supplemental Table S2). This result suggests that differences between collinear and orthogonal intact contours revealed by MVPA relate to differences in the detection ability of the observers rather than low-level stimulus differences. Further, changes in classification accuracy after training may relate to differences in the signal-to-noise ratio across sessions and areas. However, no differences were observed before and after training in the functional signal-to-noise ratio across cortical regions (Supplemental Fig. S7), suggesting that differences in the classification accuracies could not be due to differences in the overall fMRI signal across sessions. Finally, analysis of eye-movement data collected during scanning did not show any significant differences between scanning sessions (before and after training) in the eye position or number of saccades (Supplemental Fig. S8), suggesting that differences in the fMRI activation patterns before and after training could not be significantly attributed to eye-movement differences.
Taken together, these results reveal training-dependent changes in the representation of collinear versus orthogonal contours that differ across cortical areas. Training reduced the discriminability of voxel patterns responding to collinear versus orthogonal contours in early, dorsal, and temporal visual areas, suggesting that neural populations in these regions may signify the perceived similarity and enhanced detection of contour paths defined by different alignments after training. In contrast, neural populations in ventral and frontoparietal areas may represent differences in the spatial configuration of the contours (collinear vs. orthogonal alignment of the local elements) that does not change with training.
It is important to note that the multivariate effects observed are driven in most cases by strong univariate signals, as shown by significant differences between contour types when standard GLM analyses are used. Although our multivariate analyses do not provide a test of distributed representations, they provide a more sensitive tool than univariate approaches for testing training-dependent changes in the information content of voxel patterns within ROIs. In particular, the change in the information content across the voxel pattern in early, dorsal, and temporal areas may reflect enhanced neural similarity for contour types that is consistent with the observers' improved sensitivity in detecting contours in both collinear and orthogonal displays. It is possible that the same neural populations that respond to collinear contours become responsive to orthogonal contours after training or that a different set of neurons becomes sensitive to orthogonal contours. These training-dependent changes in the neural representations of contours may relate to changes in neural selectivity of single neurons or local population correlations enhanced through feedback. Although these hypotheses cannot be dissociated based on fMRI signals that represent the congregate activity of large neural populations, multivariate analyses allow us to understand these learning-dependent changes at the level of voxel patterns within ROIs.
Training-dependent changes in the spatial distribution of activation patterns
The univariate and multivariate analyses described earlier concentrated on training-dependent effects at the level of cortical regions. We further tested the effect of training on the spatial distribution of fMRI responses at the level of individual voxels. In particular, for each voxel included in the MVPA we calculated the differential fMRI response to different contour types (i.e., t-test value for collinear vs. orthogonal, collinear vs. acute, orthogonal vs. acute contours). We reasoned that correlating these values per voxel before and after training would allow us to characterize the learning-dependent changes on the spatial distribution of the activity evoked by the different contour types. In particular, high correlations would indicate that differences in the fMRI responses between contour types before training are proportional to differences in the fMRI responses after training, consistent with gain modulation effects (Op de Beeck et al. 2006). In contrast, low correlations would suggest that training changed the distribution of voxel activity. That is, the response of individual voxels for a contour type has changed (e.g., from high before training to low after training), suggesting an altered neural representation of the stimuli rather than gain modulations that would preserve the spatial distribution of activity. Rather, training modulates activity across voxels independently, possibly reflecting changed sensitivity to stimulus features in a number of voxels across the pattern.
We tested these predictions by computing t-test values expressing the difference in the fMRI responses between different contour types (collinear vs. orthogonal, orthogonal vs. acute, collinear vs. acute). We then computed correlations of these t-values per voxel before and after training (Fig. 3B). A two-way ANOVA on the correlation coefficients showed a significant interaction between contour contrasts (collinear vs. orthogonal, orthogonal vs. acute, collinear vs. acute) and ROIs [F(28,196) = 2.05, P < 0.01]. In particular, we observed high correlations across all areas for responses to collinear versus acute contours, in contrast to low correlations for responses to orthogonal versus acute contours. This is consistent with the behavioral improvement in the detection of orthogonal but not acute contours and suggests training-dependent changes in neural responses to orthogonal contours. However, correlations before and after training for responses to collinear versus orthogonal contours differed across areas when compared with responses for orthogonal versus acute contours, as shown by a significant interaction [F(14,98) = 3.18, P < 0.05] between contour contrasts and ROIs. In particular, correlation coefficients for collinear versus orthogonal contours were low and not significantly different from those for orthogonal versus acute contours in early [F(1,7) < 1, P = 0.50], ventral [F(1,7) < 1, P = 0.40], and dorsal [F(1,7) < 1, P = 0.57] visual areas. These results suggest that responses to orthogonal versus collinear contours in early, ventral, and dorsal visual areas after training did not change proportionally to responses before training. Rather, training changed the spatial distribution of activity in these areas, suggesting that the neural representation of orthogonal contours was altered. That is, voxels that before training signified differences between collinear and orthogonal contours, after training showed decreased differential responses, possibly signifying the perceived similarity of the contour path.
In summary, univariate analysis showed training-dependent changes in the magnitude of activations for collinear versus orthogonal contours in extrastriate, temporal, and frontoparietal areas. Such changes may relate to gain mechanisms that modulate cortical responsiveness based on stimulus salience. That is, responses are higher before training for collinear contours that are more salient than orthogonal contours, whereas orthogonal contours become detectable and evoke strong responses only after training. However, multivariate- and single-voxel–based analyses showed that only in early and dorsal visual areas does training change both the information content and the spatial distribution of activations, suggesting learning-dependent changes in the neural representation of orthogonal contours.
Relationship between behavioral and fMRI training-dependent changes
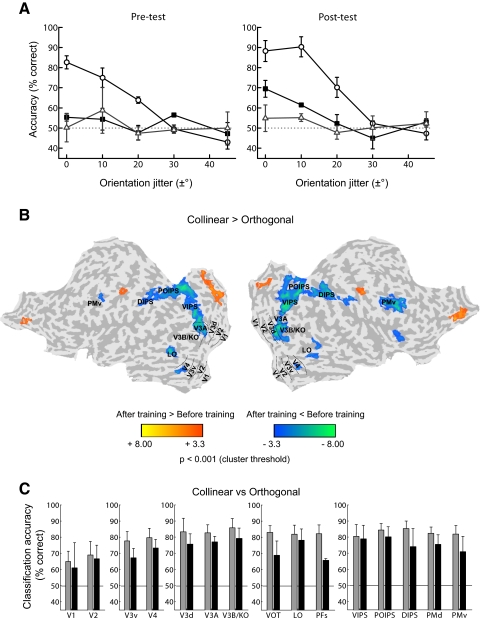
Further evidence for the relationship between behavioral and fMRI training-dependent changes comes from considering the data from three additional observers that failed to achieve the criterion detection performance after six training sessions (“weak learners”). That is, for these observers posttest detection accuracy for orthogonal contours at zero orientation jitter did not reach 75% correct. Correlating behavioral performance and fMRI responses to orthogonal contours across all observers (including both “learners” and “weak learners”) showed significant correlations in dorsal (V3d: R = 0.83, P = 0.001), temporal (LO: R = 0.70, P < 0.05), and parietal (VIPS: R = 0.63, P < 0.05; POIPS: R = 0.67, P < 0.05; DIPS: R = 0.69, P < 0.05) regions. This analysis suggests that enhanced behavioral performance in the detection of orthogonal contours was associated with increased fMRI responses after training.
The small number of “weak learners” makes the direct statistical comparison of the data from “learners” and “weak learners” difficult. However, analysis of the behavioral and fMRI data for ”weak learners” separately from the data for “learners” provides further interesting insights into the learning mechanisms for contour integration. In particular, analysis of the behavioral data (Fig. 4A) showed significant differences in performance between the stimulus conditions [F(2,4) = 24.95, P < 0.01], but lack of a training effect, as indicated by nonsignificant differences between sessions [F(1,2) = 1.79, P = 0.31] and a nonsignificant interaction between stimulus and session [F(2,4) < 1, P = 0.57]. Univariate (GLM) analysis of the fMRI data showed a pattern of results similar to that for “learners.” That is, activations for collinear versus orthogonal contours decreased significantly after training in extrastriate, temporal, and frontoparietal regions (Fig. 4B). In contrast, multivariate analyses (Fig. 4C) showed that classification accuracies for collinear versus orthogonal contours remained high after training across areas and did not differ significantly from accuracies before training across areas, as supported by a nonsignificant interaction between session and ROI [F(14,28) < 1, P = 0.59]. These high classification accuracies for collinear versus orthogonal contours were potentially due to higher fMRI signals for collinear contours that were more salient than orthogonal contours both before and after training for weak learners. Thus training did not change the neural similarity between collinear and orthogonal contours for “weak learners,” consistent with the lack of changes in their behavioral sensitivity for the detection of orthogonal contours after training.
FIG. 4.
Behavioral and fMRI data for “weak learners.” A: psychometric curves for “weak learners” (average across observers, n = 3) for contour detection performance (percentage correct) plotted as a function of local orientation jitter before training (pretest) and after training (posttest). Circles: collinear contours; squares: orthogonal contours; triangles: acute contours. Error bars denote ±SE across observers. B: group GLM maps showing significant differences between sessions (before and after training) for the differential fMRI response to collinear vs. orthogonal contours. Data are presented on a flattened reconstruction of 2 cortical hemispheres. Negative t-values indicate that the difference in responses to collinear vs. orthogonal contours after training was lower than that before training (P < 0.001, cluster threshold correction). Dotted lines indicate the borders between retinotopic visual areas. C: MVPA accuracy (percentage correct) per ROI (voxel-pattern size of 152 voxels) for classification between collinear and orthogonal contours. Gray bars: before training; black bars: after training. Error bars denote ±SE across observers.
These results suggest that the changes in the magnitude of the fMRI responses revealed by the univariate analyses for both “learners” and “weak learners” may be due to repeated exposure to the stimuli throughout training that modulates neural signals related to stimulus salience and familiarity. In contrast, changes in the information content of voxel patterns as revealed by MVPA in early visual, dorsal, and temporal areas for “learners” rather than “weak learners” suggests training-dependent changes in the neural representation of the trained stimulus that relate to changes in behavioral sensitivity rather than simply repeated exposure to the stimulus.
Attentional modulation versus training-dependent neural changes
Is it possible that the fMRI changes observed after training could be simply due to differential allocation of attention to the different contour types across sessions (before vs. after training)? This attentional modulation hypothesis could not explain the lack of differences in the classification accuracies before and after training in ventral and frontoparietal regions that are known to be highly involved in attentional processing.
To control for this possibility, we tested the effect of training on the neural representation of a stimulus feature (i.e., contour orientation) that is common for collinear and orthogonal contours. In a control experiment, observers (n = 4) were trained with the same procedure as that in the main experiment but when tested in the scanner they were presented with collinear and orthogonal stimuli blocked by the contour orientation: near the left (135 ± 5°) versus right diagonal (45 ± 5°). To further control for attentional effects observers performed a dual task across all stimulus conditions—that is, rather than detecting only collinear contours in cardinal orientations (as in the main experiment), observers were also required to detect a change in the shape of the fixation cross. Observers performed similarly well in both tasks across conditions and sessions, suggesting similar attentional allocation. That is, a repeated-measures ANOVA showed no significant differences between sessions (i.e., before and after training) [cardinal detection: F(1,3) = 1.3, P = 0.34; fixation task: F(1,3) = 1.3, P = 0.34] or conditions [cardinal detection: F(5,15) < 1, P = 0.52; fixation task: F(6,18) < 1, P = 0.99].
Analysis of the behavioral data showed results similar to those for the main experiment; that is, training on orthogonal contours resulted in enhanced detection performance [F(1,3) = 22.7, P < 0.05]. GLM analysis (Fig. 5A) showed activation patterns similar to those in the main experiment. That is, activations in ventral, dorsal, temporal, and intraparietal areas for collinear versus orthogonal contours decreased after training. Further pattern classification on fMRI responses to left versus right contour orientations in retinotopic visual areas known to have orientation-selective neurons showed that these areas contained reliable information for decoding the orientation of orthogonal contours only after training (Fig. 5B). That is, classification accuracies increased after training (compared with chance levels before training) in extrastriate areas but not V1 [t(3) = 0.87, P = 0.45]. In contrast, classification of orientations for collinear contours was reliable both before and after training and no significant differences in the accuracies across areas were observed between scanning sessions [F(1,3) = 2.5, P = 0.21]. These results suggest that neural populations in extrastriate visual areas could reliably discriminate between orientations only when orthogonal contours were rendered salient by training. The lack of significant effects in V1 could be due to the fixation task demands that may have withdrawn attention from the stimulus, consistent with previous physiological findings showing reduced training-dependent V1 modulations for a dimming compared with a contour-detection task (Li et al. 2008). In sum, these findings support training-dependent changes in the neural representation of contour features (i.e., orientation) in extrastriate visual areas that could not be simply explained by differential allocation of attention to the stimuli across scanning sessions because the two contour orientations tested were equivalent in salience within each scanning session.
FIG. 5.
Control experiment. A: group GLM maps showing significant differences between sessions (before and after training) for the differential fMRI response to collinear vs. orthogonal contours. Data are presented on a flattened reconstruction of 2 cortical hemispheres. Negative t-values indicate that the difference in responses to collinear vs. orthogonal contours after training was lower than that before training (P < 0.001, cluster threshold correction). Dotted lines indicate the borders between retinotopic visual areas. B: MVPA accuracy (percentage correct) per ROI (voxel-pattern size of 120 voxels) for classification of the orientation [near the left (135 ± 5°) vs. right diagonal (45 ± 5°)] of orthogonal contours before and after training. Gray bars: before training; black bars: after training. Error bars denote ±SE across observers.
DISCUSSION
Our findings demonstrate that experience shapes the neural processing of camouflaged targets by enhancing neural sensitivity primarily in dorsal visual areas to image regularities that are behaviorally relevant for target detection, albeit atypical of natural contours. Specifically, collinear and orthogonal contours share similar local images statistics (i.e., elements of the same orientation co-occur at different alignments relative to the contour paths). However, consistent with previous studies (Bex et al. 2001; Field et al. 1993), initially observers were able to reliably detect only collinear contours. Only after training (2,400–3,600 trials, over four to six sessions) did observers reach similar levels of performance for detecting orthogonal as for collinear contours. This finding replicates our previous behavioral studies (Schwarzkopf and Kourtzi 2008) showing that experience shapes the utility of image regularities and enhances the observers' ability to exploit orthogonal alignments that typically signify discontinuities for the detection of continuities in contour paths. Importantly, by combining behavioral and imaging measurements, we provide novel evidence for two signatures of cortical plasticity that mediate this flexible learning of image regularities for contour detection. In particular, training results in changes in the responsiveness of an extensive occipitotemporal and frontoparietal network of areas. That is, univariate analyses show changes in fMRI magnitude expressed by increased responses for orthogonal contours but decreased responses for collinear contours after training. These changes in the fMRI response magnitude suggest a gain mechanism related to stimulus salience and familiarity that could be triggered by frontoparietal regions and thus modulate the processing of occipitotemporal areas. In contrast, primarily in dorsal visual areas, learning changes neural sensitivity to reflect the enhanced behavioral sensitivity to the global contour path and the perceptual similarity between contours defined by different alignments. In particular, multivariate- and single-voxel–based analyses reveal that experience modulates the information content and spatial distribution of voxels in these areas to reflect neural similarity between collinear and orthogonal contours rather than simply gain modulations of activity.
Our findings advance our understanding of experience-dependent plasticity mechanisms for contour detection in the following main respects. First, previous psychophysical studies have shown that learning enhances the ability of observers to detect targets in noise (Brady and Kersten 2003; Dosher and Lu 1998; Eckstein et al. 2004; Fahle 2004; Fine and Jacobs 2002; Furmanski and Engel 2000; Gilbert et al. 2001; Gold et al. 1999; Kovacs et al. 1999; Li and Gilbert 2002; Li RW et al. 2004; Polat and Sagi 1994; Sagi and Tanne 1994; Sigman and Gilbert 2000). Further, previous imaging studies have shown that learning changes cortical responses in accordance with the level of behavioral improvement (Dolan et al. 1997; Gauthier et al. 1999; Grill-Spector et al. 2000; Kourtzi et al. 2005; Mukai et al. 2007; Op de Beeck et al. 2006; Schwartz et al. 2002; Sigman et al. 2005; Yotsumoto et al. 2008). However, our study focuses on how learning determines the principles that facilitate contour linking in cluttered scenes. Our findings show that short-term learning shapes the functional optimization of visual recognition processes by altering the neural representation of behaviorally relevant image regularities that violate the typical principles of contour linking (Geisler 2008; Sigman et al. 2001; Simoncelli and Olshausen 2001). Although collinearity is a prevalent principle for perceptual integration in natural scenes, we show that the brain learns to exploit other image regularities (i.e., orthogonal alignments) that typically signify discontinuities for contour linking. Further studies are needed to investigate the neural mechanisms that support learning of different types of image regularities. For example, our previous behavioral studies (Schwarzkopf and Kourtzi 2008) showed that learning to exploit image regularities for contour linking is slower and weaker for acute than that for orthogonal alignments. Previous studies implicating both excitatory and inhibitory mechanisms for contour integration and surface segmentation (Kapadia et al. 2000; Knierim and van Essen 1992; Li 1998; Ursino and La Cara 2004; Yen and Finkel 1998) provide insights in understanding these differences. It is possible that orthogonal alignments are more effective cues for segmentation than acute ones because elements at right angle to the contour path fall within the inhibitory flanking side bands of neurons that may support surface segmentation, whereas elements at acute angles fall in between the facilitatory regions that may support collinear correlations and the inhibitory regions of the surround. Our previous behavioral findings suggest that in naïve observers longer training is necessary for boosting these weak associations between acute elements than the amount of training sufficient for linking and detecting orthogonal contours.
Second, previous imaging studies searching for the neural signatures of visual learning in the human brain have reported changes (increases or decreases) in the magnitude of fMRI activity (Kourtzi et al. 2005; Mukai et al. 2007; Schwartz et al. 2002; Sigman et al. 2005; Yotsumoto et al. 2008). These fMRI training effects may relate to gain changes that modulate the magnitude of neural responses or changes in neuronal tuning that result in enhanced selectivity for the relevant stimulus features in a smaller neural population and a decrease of the average fMRI signal across populations within a given region. Enhanced fMRI responses have been associated with increased neural recruitment when the sensory input is ambiguous and the task difficult, whereas decreased responses have been observed when learning has been consolidated, the sensory input becomes more salient, and the task easier. Comparing univariate and multivariate analyses of fMRI signals, our study discerns the role of these mechanisms in learning image regularities for contour detection. We show that gain modulations in a large network of areas (higher occipitotemporal and frontoparietal regions) may support the detection of salient regions (e.g., regions comprising local elements of similar alignment). Such changes are similar for “learners” and “weak learners,” suggesting that gain changes may be simply due to repeated exposure to the trained stimuli rather than reflecting training-dependent changes in visual sensitivity. In contrast, changes in the information content and spatial distribution of voxels primarily in dorsal visual areas, as revealed by multivoxel pattern analysis, reflect changes in neural sensitivity for the global contour. Three lines of evidence support the link between training-specific changes in behavioral and neural sensitivity. First, changes in the multivoxel patterns were evident when training resulted in enhanced perceptual sensitivity for the detection of orthogonal contours in “learners” rather than “weak learners.” Second, classification of fMRI signals evoked by different contour orientations was reliable only after training, suggesting that training enhances neural sensitivity to the relevant global contour features (i.e., orientation) in extrastriate visual areas. Finally, these changes in neural sensitivity were measured while observers engaged in attentionally demanding tasks, suggesting that the neural representations of the orthogonal contours have been altered through training and reflect the observers' experience rather than transient task-specific attentional modulation.
Third, our findings provide evidence that the optimization of perceptual integration and contour detection processes through learning may entail recurrent processing between local integration mechanisms and top-down influences. Consistent with a theoretical model of attention-gated reinforcement learning (Roelfsema and van Ooyen 2005), the gain effects observed across cortical circuits may relate to a global reinforcement mechanism that is important for identifying salient image regions with local elements of similar alignment. Goal-directed attentional mechanisms may then optimize visual processing within these salient image regions and change the neural sensitivity to the relevant contour features for the detection of contour paths rather than spurious image correlations (Itti and Koch 2001; Navalpakkam and Itti 2007; Treue 2003). Our findings show that fMRI responses after training were consistent with a change in the overall perceptual salience of the orthogonal contours in intraparietal (VIPS, POIPS, DIPS) and premotor areas. These areas are thought to be involved in the allocation of visual attention and in generating salience maps based on stimulus features and action goals (Colby and Goldberg 1999; Ipata et al. 2006). Attentional processing in these areas may modulate processing in visual areas by changing their responsiveness (i.e., gain changes) (McAdams and Maunsell 2000; Reynolds and Chelazzi 2004; Reynolds et al. 2000; Saenz et al. 2002; Treue and Martinez Trujillo 1999). In contrast, changes of neural representations primarily in dorsal visual areas may relate to changes in neural selectivity or local correlations involved in the integration and processing of forms (Ostwald et al. 2008). Recent neurophysiological studies propose that learning may support efficient target detection (Barlow 1990) by enhancing the salience of targets through increased correlation of neuronal signals related to the target features and decorrelation of signals related to target and background features (Jagadeesh et al. 2001; Li W et al. 2008). Such signal correlations could be enhanced by recurrent processes involving feedback from higher frontoparietal regions and improved attention to the relevant features for contour detection.
Characterizing these interactions and direction of information flow between frontoparietal circuits selecting salient regions and occipitotemporal regions involved in the integration and detection of contours is difficult with fMRI measurements alone due to limited temporal resolution. However, recent physiological findings provide insights into these interactions. Recurrent processing involving intrinsic connections between neurons with similar orientation preference (Bosking et al. 1997; Chisum and Fitzpatrick 2004; Gilbert and Wiesel 1989; Malach et al. 1993) and feedback from higher visual areas (Fitzpatrick 2000; Gilbert and Wiesel 1989; Gilbert et al. 2001; Hochstein and Ahissar 2002; Sigman et al. 2001) has been suggested to facilitate perceptual integration and figure–ground segmentation as early as in V1 (Roelfsema 2006; Zipser et al. 1996). The evidence on experience-dependent plasticity in V1 is controversial. Consistent with previous neurophysiological findings showing that learning does not alter the topography or basic receptive field properties (e.g., size, location, orientation selectivity) in V1 (Crist et al. 2001; Ghose et al. 2002), we did not observe significant changes in fMRI responses to orthogonal contours after training in V1. However, changes in the voxel pattern in V1 indicated changes in neural sensitivity after training that may correspond to enhanced perceptual sensitivity for orthogonal contours. These findings are consistent with previous imaging studies showing enhanced responses in V1 for oblique orientations after training (Furmanski et al. 2004) and physiology showing changes in orientation tuning (Schoups et al. 2001). However, training-dependent changes on orientation tuning are shown to be more pronounced in V4 (Raiguel et al. 2006; Yang and Maunsell 2004), whereas effects in V1 are shown to be task dependent and may engage top-down facilitation mechanisms (Crist et al. 2001; Li W et al. 2004, 2008; Sigman et al. 2005). For example, recent physiological studies show training-dependent modulation of V1 responses for collinear contours in accordance with the perceptual salience of these contours (Li et al. 2008). Such modulations were observed only after training, were absent under anesthesia, and were reduced by tasks diverting attention away from the stimulus, suggesting feedback influences. Consistent with these findings, we did not observe any significant changes in sensitivity to contour orientation when observers performed a dual-attention task. Taken together these findings suggest that attention-gated top-down mechanisms may modulate responses in V1 during training in a task-dependent manner (Crist et al. 2001; Li W et al. 2004) by changing read-out signals rather than the neural encoding (Law and Gold 2008).
Conclusions
Evolution and long-term experience during development have been suggested to shape the neural architecture of the visual cortex in a manner that resembles the geometry of natural scenes (Geisler 2008; Gilbert 1992; Kovacs et al. 1999; Sigman et al. 2001; Simoncelli and Olshausen 2001). Early and long-term experience with the high prevalence of collinear edges in natural environments (Geisler et al. 2001; Sigman et al. 2001) may shape long-range connections between primary visual cortex neurons with similar orientation preferences. Although our study did not directly test the role of evolution, genetics, or developmental plasticity in shaping the mechanisms of contour integration, our findings provide novel evidence that short-term experience in adulthood may modulate the function of these connections and the signals they integrate. In particular, training changes the neural sensitivity to image regularities that define discontinuities and are atypical for contours in natural scenes. These experience-dependent changes in neural sensitivity—in line with improved perceptual sensitivity for the detection of orthogonal contours—are evident primarily in higher dorsal and temporal visual areas that are known to be involved in the integration of shapes. Gain changes in the magnitude of fMRI responses in frontoparietal regions signify changes in stimulus salience that may guide the adaptive reorganization of connections as early as in primary visual cortex in a top-down manner. Thus our findings demonstrate that experience at shorter timescales in adulthood plays an important role in the adaptive functional optimization of the visual system for the perceptual interpretation of natural scenes. Determining whether the mechanisms that mediate learning in adulthood are the same as those operating through evolution and early development remains a challenge for future work.
GRANTS
This work was supported by Biotechnology and Biological Sciences Research Council Grant BB/D52199X/1 and Cognitive Foresight Initiative Grant BB/E027436/1 to Z. Kourtzi.
Supplementary Material
Acknowledgments
We thank M. Dexter for help with analysis of the eye movement data.
Footnotes
The online version of this article contains supplemental data.
REFERENCES
- Barlow 1990.Barlow H. Conditions for versatile learning, Helmholtz's unconscious inference, and the task of perception. Vision Res 30: 1561–1571, 1990. [DOI] [PubMed] [Google Scholar]
- Bex 2001.Bex PJ, Simmers AJ, Dakin SC. Snakes and ladders: the role of temporal modulation in visual contour integration. Vision Res 41: 3775–3782, 2001. [DOI] [PubMed] [Google Scholar]
- Bosking 1997.Bosking WH, Zhang Y, Schofield B, Fitzpatrick D. Orientation selectivity and the arrangement of horizontal connections in tree shrew striate cortex. J Neurosci 17: 2112–2127, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady 2003.Brady MJ, Kersten D. Bootstrapped learning of novel objects. J Vis 3: 413–422, 2003. [DOI] [PubMed] [Google Scholar]
- Chisum 2004.Chisum HJ, Fitzpatrick D. The contribution of vertical and horizontal connections to the receptive field center and surround in V1. Neural Netw 17: 681–693, 2004. [DOI] [PubMed] [Google Scholar]
- Colby 1999.Colby CL, Goldberg ME. Space and attention in parietal cortex. Annu Rev Neurosci 22: 319–349, 1999. [DOI] [PubMed] [Google Scholar]
- Cox 2003.Cox DD, Savoy RL. Functional magnetic resonance imaging (fMRI) “brain reading”: detecting and classifying distributed patterns of fMRI activity in human visual cortex. NeuroImage 19: 261–270, 2003. [DOI] [PubMed] [Google Scholar]
- Crist 2001.Crist RE, Li W, Gilbert CD. Learning to see: experience and attention in primary visual cortex. Nat Neurosci 4: 519–525, 2001. [DOI] [PubMed] [Google Scholar]
- Dakin 1997.Dakin SC, Hess RF. The spatial mechanisms mediating symmetry perception. Vision Res 37: 2915–2930, 1997. [DOI] [PubMed] [Google Scholar]
- DeYoe 1996.DeYoe EA, Carman GJ, Bandettini P, Glickman S, Wieser J, Cox R, Miller D, Neitz J. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc Natl Acad Sci USA 93: 2382–2386, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan 1997.Dolan RJ, Fink GR, Rolls E, Booth M, Holmes A, Frackowiak RS, Friston KJ. How the brain learns to see objects and faces in an impoverished context. Nature 389: 596–599, 1997. [DOI] [PubMed] [Google Scholar]
- Dosher 1998.Dosher BA, Lu ZL. Perceptual learning reflects external noise filtering and internal noise reduction through channel reweighting. Proc Natl Acad Sci USA 95: 13988–13993, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckstein 2004.Eckstein MP, Abbey CK, Pham BT, Shimozaki SS. Perceptual learning through optimization of attentional weighting: human versus optimal Bayesian learner. J Vis 4: 1006–1019, 2004. [DOI] [PubMed] [Google Scholar]
- Elder 2002.Elder JH, Goldberg RM. Ecological statistics of Gestalt laws for the perceptual organization of contours. J Vis 2: 324–353, 2002. [DOI] [PubMed] [Google Scholar]
- Engel 1994.Engel SA, Rumelhart DE, Wandell BA, Lee AT, Glover GH, Chichilnisky E-J, Shadlen MN. fMRI of human visual cortex (Letter). Nature 369: 525, 1994. [DOI] [PubMed] [Google Scholar]
- Fahle 2004.Fahle M. Perceptual learning: a case for early selection. J Vis 4: 879–890, 2004. [DOI] [PubMed] [Google Scholar]
- Field 1993.Field DJ, Hayes A, Hess RF. Contour integration by the human visual system: evidence for a local “association field.” Vision Res 33: 173–193, 1993. [DOI] [PubMed] [Google Scholar]
- Fine 2002.Fine I, Jacobs RA. Comparing perceptual learning tasks: a review. J Vis 2: 190–203, 2002. [DOI] [PubMed] [Google Scholar]
- Fink 1996.Fink GR, Halligan PW, Marshall JC, Frith CD, Frackowiak RS, Dolan RJ. Where in the brain does visual attention select the forest and the trees? Nature 382: 626–628, 1996. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick 2000.Fitzpatrick D. Seeing beyond the receptive field in primary visual cortex. Curr Opin Neurobiol 10: 438–443, 2000. [DOI] [PubMed] [Google Scholar]
- Furmanski 2000.Furmanski CS, Engel SA. Perceptual learning in object recognition: object specificity and size invariance. Vision Res 40: 473–484, 2000. [DOI] [PubMed] [Google Scholar]
- Furmanski 2004.Furmanski CS, Schluppeck D, Engel SA. Learning strengthens the response of primary visual cortex to simple patterns. Curr Biol 14: 573–578, 2004. [DOI] [PubMed] [Google Scholar]
- Gauthier 1999.Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC. Activation of the middle fusiform “face area” increases with expertise in recognizing novel objects. Nat Neurosci 2: 568–573, 1999. [DOI] [PubMed] [Google Scholar]
- Geisler 2008.Geisler WS. Visual perception and the statistical properties of natural scenes. Annu Rev Psychol 59: 167–192, 2008. [DOI] [PubMed] [Google Scholar]
- Geisler 2001.Geisler WS, Perry JS, Super BJ, Gallogly DP. Edge co-occurrence in natural images predicts contour grouping performance. Vision Res 41: 711–724, 2001. [DOI] [PubMed] [Google Scholar]
- Ghose 2002.Ghose GM, Yang T, Maunsell JH. Physiological correlates of perceptual learning in monkey V1 and V2. J Neurophysiol 87: 1867–1888, 2002. [DOI] [PubMed] [Google Scholar]
- Gilbert 1992.Gilbert CD. Horizontal integration and cortical dynamics. Neuron 9: 1–13, 1992. [DOI] [PubMed] [Google Scholar]
- Gilbert 2001.Gilbert CD, Sigman M, Crist RE. The neural basis of perceptual learning. Neuron 31: 681–697, 2001. [DOI] [PubMed] [Google Scholar]
- Gilbert 1989.Gilbert CD, Wiesel TN. Columnar specificity of intrinsic horizontal and corticocortical connections in cat visual cortex. J Neurosci 9: 2432–2442, 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold 1999.Gold J, Bennett PJ, Sekuler AB. Signal but not noise changes with perceptual learning. Nature 402: 176–178, 1999. [DOI] [PubMed] [Google Scholar]
- Grill-Spector 2000.Grill-Spector K, Kushnir T, Hendler T, Malach R. The dynamics of object-selective activation correlate with recognition performance in humans. Nat Neurosci 3: 837–843, 2000. [DOI] [PubMed] [Google Scholar]
- Haynes 2005.Haynes JD, Rees G. Predicting the orientation of invisible stimuli from activity in human primary visual cortex. Nat Neurosci 8: 686–691, 2005. [DOI] [PubMed] [Google Scholar]
- Haynes 2006.Haynes JD, Rees G. Decoding mental states from brain activity in humans. Nat Rev 7: 523–534, 2006. [DOI] [PubMed] [Google Scholar]
- Hess 1999.Hess R, Field D. Integration of contours: new insights. Trends Cogn Sci 3: 480–486, 1999. [DOI] [PubMed] [Google Scholar]
- Hess 2000.Hess RF, Ledgeway T, Dakin S. Impoverished second-order input to global linking in human vision. Vision Res 40: 3309–3318, 2000. [DOI] [PubMed] [Google Scholar]
- Hochstein 2002.Hochstein S, Ahissar M. View from the top: hierarchies and reverse hierarchies in the visual system. Neuron 36: 791–804, 2002. [DOI] [PubMed] [Google Scholar]
- Ipata 2006.Ipata AE, Gee AL, Gottlieb J, Bisley JW, Goldberg ME. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat Neurosci 9: 1071–1076, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itti 2001.Itti L, Koch C. Computational modelling of visual attention. Nat Rev 2: 194–203, 2001. [DOI] [PubMed] [Google Scholar]
- Jagadeesh 2001.Jagadeesh B, Chelazzi L, Mishkin M, Desimone R. Learning increases stimulus salience in anterior inferior temporal cortex of the macaque. J Neurophysiol 86: 290–303, 2001. [DOI] [PubMed] [Google Scholar]
- Kamitani 2005.Kamitani Y, Tong F. Decoding the visual and subjective contents of the human brain. Nat Neurosci 8: 679–685, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapadia 2000.Kapadia MK, Westheimer G, Gilbert CD. Spatial distribution of contextual interactions in primary visual cortex and in visual perception. J Neurophysiol 84: 2048–2062, 2000. [DOI] [PubMed] [Google Scholar]
- Knierim 1992.Knierim JJ, van Essen DC. Neuronal responses to static texture patterns in area V1 of the alert macaque monkey. J Neurophysiol 67: 961–980, 1992. [DOI] [PubMed] [Google Scholar]
- Kourtzi 2005.Kourtzi Z, Betts LR, Sarkheil P, Welchman AE. Distributed neural plasticity for shape learning in the human visual cortex (Abstract). PLoS Biol 3: e204, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs 1999.Kovacs I, Kozma P, Feher A, Benedek G. Late maturation of visual spatial integration in humans. Proc Natl Acad Sci USA 96: 12204–12209, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger 2002.Kruger N, Worgotter F. Multi-modal estimation of collinearity and parallelism in natural image sequences. Network (Bristol, England) 13: 553–576, 2002. [PubMed] [Google Scholar]
- Kuai 2005.Kuai SG, Zhang JY, Klein SA, Levi DM, Yu C. The essential role of stimulus temporal patterning in enabling perceptual learning. Nat Neurosci 8: 1497–1499, 2005. [DOI] [PubMed] [Google Scholar]
- Law 2008.Law CT, Gold JI. Neural correlates of perceptual learning in a sensory-motor, but not a sensory, cortical area. Nat Neurosci 11: 505–513, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledgeway 2005.Ledgeway T, Hess RF, Geisler WS. Grouping local orientation and direction signals to extract spatial contours: empirical tests of “association field” models of contour integration. Vision Res 45: 2511–2522, 2005. [DOI] [PubMed] [Google Scholar]
- Li 2004.Li RW, Levi DM, Klein SA. Perceptual learning improves efficiency by re-tuning the decision “template” for position discrimination. Nat Neurosci 7: 178–183, 2004. [DOI] [PubMed] [Google Scholar]
- Li 2002.Li W, Gilbert CD. Global contour saliency and local colinear interactions. J Neurophysiol 88: 2846–2856, 2002. [DOI] [PubMed] [Google Scholar]
- Li 2004.Li W, Piëch V, Gilbert CD. Perceptual learning and top-down influences in primary visual cortex. Nat Neurosci 7: 651, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li 2008.Li W, Piëch V, Gilbert CD. Learning to link visual contours. Neuron 57: 442–451, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li 1998.Li Z. A neural model of contour integration in the primary visual cortex. Neural Comput 10: 903–940, 1998. [DOI] [PubMed] [Google Scholar]
- Malach 1993.Malach R, Amir Y, Harel M, Grinvald A. Relationship between intrinsic connections and functional architecture revealed by optical imaging and in vivo targeted biocytin injections in primate striate cortex. Proc Natl Acad Sci USA 90: 10469–10473, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdams 2000.McAdams CJ, Maunsell JH. Attention to both space and feature modulates neuronal responses in macaque area V4. J Neurophysiol 83: 1751–1755, 2000. [DOI] [PubMed] [Google Scholar]
- Mukai 2007.Mukai I, Kim D, Fukunaga M, Japee S, Marrett S, Ungerleider LG. Activations in visual and attention-related areas predict and correlate with the degree of perceptual learning. J Neurosci 27: 11401–11411, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navalpakkam 2007.Navalpakkam V, Itti L. Search goal tunes visual features optimally. Neuron 53: 605–617, 2007. [DOI] [PubMed] [Google Scholar]
- Norman 2006.Norman KA, Polyn SM, Detre GJ, Haxby JV. Beyond mind-reading: multi-voxel pattern analysis of fMRI data. Trends Cogn Sci 10: 424–430, 2006. [DOI] [PubMed] [Google Scholar]
- Op de Beeck 2006.Op de Beeck HP, Baker CI, DiCarlo JJ, Kanwisher NG. Discrimination training alters object representations in human extrastriate cortex. J Neurosci 26: 13025–13036, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostwald 2008.Ostwald D, Lam JM, Li S, Kourtzi Z. Neural coding of global form in the human visual cortex. J Neurophysiol 99: 2456–2469, 2008. [DOI] [PubMed] [Google Scholar]
- Polat 1994.Polat U, Sagi D. Spatial interactions in human vision: from near to far via experience-dependent cascades of connections. Proc Natl Acad Sci USA 91: 1206–1209, 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiguel 2006.Raiguel S, Vogels R, Mysore SG, Orban GA. Learning to see the difference specifically alters the most informative V4 neurons. J Neurosci 26: 6589–6602, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds 2004.Reynolds JH, Chelazzi L. Attentional modulation of visual processing. Annu Rev Neurosci 27: 611–647, 2004. [DOI] [PubMed] [Google Scholar]
- Reynolds 2000.Reynolds JH, Pasternak T, Desimone R. Attention increases sensitivity of V4 neurons. Neuron 26: 703–714, 2000. [DOI] [PubMed] [Google Scholar]
- Roelfsema 2006.Roelfsema PR. Cortical algorithms for perceptual grouping. Annu Rev Neurosci 29: 203–227, 2006. [DOI] [PubMed] [Google Scholar]
- Roelfsema 2005.Roelfsema PR, van Ooyen A. Attention-gated reinforcement learning of internal representations for classification. Neural Comput 17: 2176–2214, 2005. [DOI] [PubMed] [Google Scholar]
- Saenz 2002.Saenz M, Buracas GT, Boynton GM. Global effects of feature-based attention in human visual cortex. Nat Neurosci 5: 631–632, 2002. [DOI] [PubMed] [Google Scholar]
- Sagi 1994.Sagi D, Tanne D. Perceptual learning: learning to see. Curr Opin Neurobiol 4: 195–199, 1994. [DOI] [PubMed] [Google Scholar]
- Schoups 2001.Schoups A, Vogels R, Qian N, Orban G. Practising orientation identification improves orientation coding in V1 neurons. Nature 412: 549–553, 2001. [DOI] [PubMed] [Google Scholar]
- Schwartz 2002.Schwartz S, Maquet P, Frith C. Neural correlates of perceptual learning: a functional MRI study of visual texture discrimination. Proc Natl Acad Sci USA 99: 17137–17142, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzkopf 2008.Schwarzkopf DS, Kourtzi Z. Experience shapes the utility of natural statistics for perceptual contour integration. Curr Biol 18: 1162–1167, 2008. [DOI] [PubMed] [Google Scholar]
- Sereno 1995.Sereno MI, Dale AM, Reppas JB, Kwong KK, Belliveau JW, Brady TJ, Rosen BR, Tootell RB. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268: 889–893, 1995. [DOI] [PubMed] [Google Scholar]
- Sigman 2001.Sigman M, Cecchi GA, Gilbert CD, Magnasco MO. On a common circle: natural scenes and Gestalt rules. Proc Natl Acad Sci USA 98: 1935–1940, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman 2000.Sigman M, Gilbert CD. Learning to find a shape. Nat Neurosci 3: 264–269, 2000. [DOI] [PubMed] [Google Scholar]
- Sigman 2005.Sigman M, Pan H, Yang Y, Stern E, Silbersweig D, Gilbert CD. Top-down reorganization of activity in the visual pathway after learning a shape identification task. Neuron 46: 823–835, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncelli 2001.Simoncelli EP, Olshausen BA. Natural image statistics and neural representation. Annu Rev Neurosci 24: 1193–1216, 2001. [DOI] [PubMed] [Google Scholar]
- Treue 2003.Treue S. Visual attention: the where, what, how and why of saliency. Curr Opin Neurobiol 13: 428–432, 2003. [DOI] [PubMed] [Google Scholar]
- Treue 1999.Treue S, Martinez Trujillo JC. Feature-based attention influences motion processing gain in macaque visual cortex. Nature 399: 575–579, 1999. [DOI] [PubMed] [Google Scholar]
- Ursino 2004.Ursino M, La Cara GE. A model of contextual interactions and contour detection in primary visual cortex. Neural Netw 17: 719–735, 2004. [DOI] [PubMed] [Google Scholar]
- Yang 2004.Yang T, Maunsell JH. The effect of perceptual learning on neuronal responses in monkey visual area V4. J Neurosci 24: 1617–1626, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen 1998.Yen SC, Finkel LH. Extraction of perceptually salient contours by striate cortical networks. Vision Res 38: 719–741, 1998. [DOI] [PubMed] [Google Scholar]
- Yotsumoto 2008.Yotsumoto Y, Watanabe T, Sasaki Y. Different dynamics of performance and brain activation in the time course of perceptual learning. Neuron 57: 827–833, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipser 1996.Zipser K, Lamme VA, Schiller PH. Contextual modulation in primary visual cortex. J Neurosci 16: 7376–7389, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.