Figure 1.
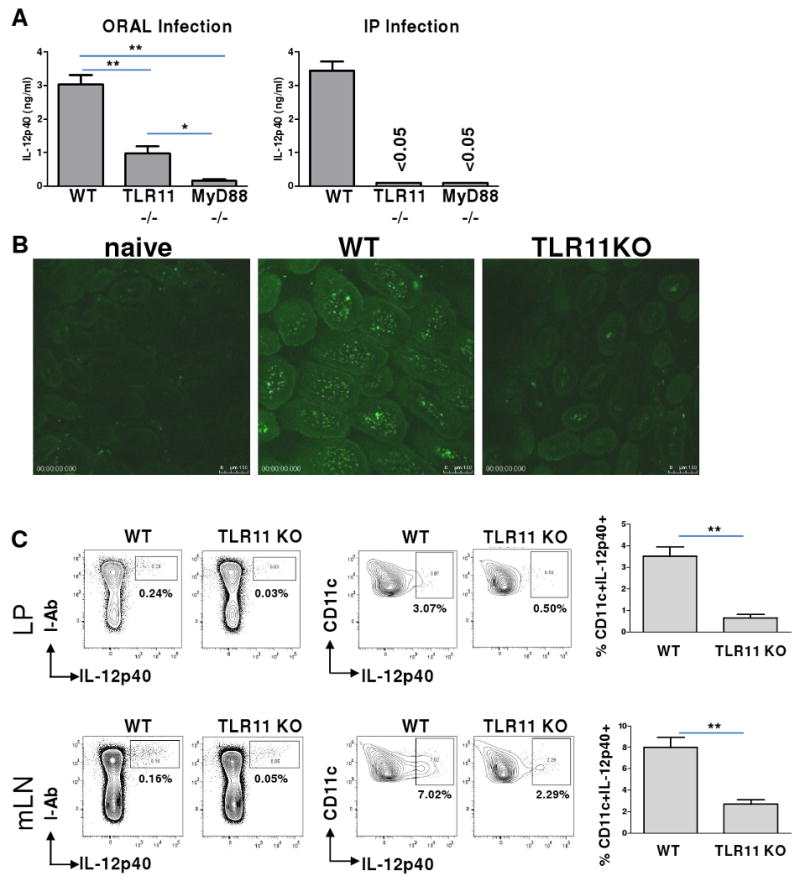
TLR11 is essential for in vivo DC-IL-12 responses to T. gondii during systemic infection, but is dispensable for mucosal immune responses to the parasite.
(A) WT, TLR11-/-, and MyD88–/– mice (five animals per group) were infected orally or intraperitoneally (IP) with an average of 20 T. gondii ME49 strain cysts per mouse. Serum IL-12p40 responses were measured 5 days later by ELISA. The data shown are the mean ± SD; the results are representative of four independent experiments. (B) Visualization of IL-12p40-producing cells during oral infection with T. gondii. WT IL-12p40-YFP reporter animals (Yet40) or TLR11-/-×Yet40 mice were infected orally with T. gondii, as described above. Small intestines were removed for microscopic analysis on day 5 post-infection to visualize IL-12p40-producing cells. Images were acquired with a Leica SPE with a 63× objective. The experiment shown is representative of the twelve performed. (C) DCs are the major IL-12-producing cells in WT and TLR11-/- mice. The different mucosal cell populations in the lamina propria (LP) of the small intestine and in the mLNs were examined for their ability to produce IL-12 in response to T. gondii. WT Yet40 and TLR11-/-×Yet40 mice were infected orally with T. gondii, as described above. On day 5 post-infection, small intestine and mLN single cell suspensions were prepared, stained with antibodies to I-Ab, CD11c, CD11b, and CD8α cell surface markers, and analyzed using flow cytometry. The data shown are the mean ± SD; the results are representative of four independent experiments, each involving four or five animals per group. * P< 0.05; ** P< 0.01.
