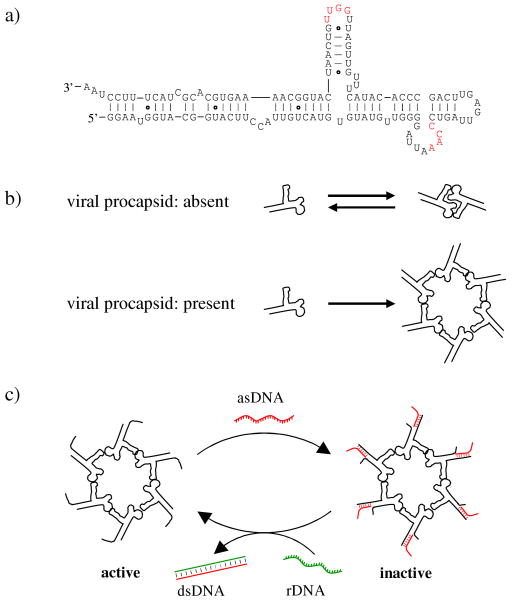
Figure 1.
Reversible switching of the biological activity of the hexameric pRNA complex of bacteriophage phi29 DNA packaging motor. (a) The secondary structure of the native pRNA molecule. The 3′ end of pRNA has been elongated in the current study to facilitate the asDNA binding. The two interacting loops are colored red. pRNA: packaging RNA; asDNA: antisense DNA; rDNA: removal DNA. (b) pRNA self-association. In the absence of viral procapsids, pRNAs reversibly associate with each other into dimers. The equilibrium prefers pRNA monomer when the interacting loop sequences are as shown in (a). No higher oligomeric species can form. In the presence of procapsids, pRNA interacts with procapsids and forms hexameric rings around the packaging porters. (c) Regulation of the pRNA function by reversible and isothermal binding/removal of asDNA. Note that it is not necessary for asDNA to bind to all pRNA to inhibit the packaging motor.