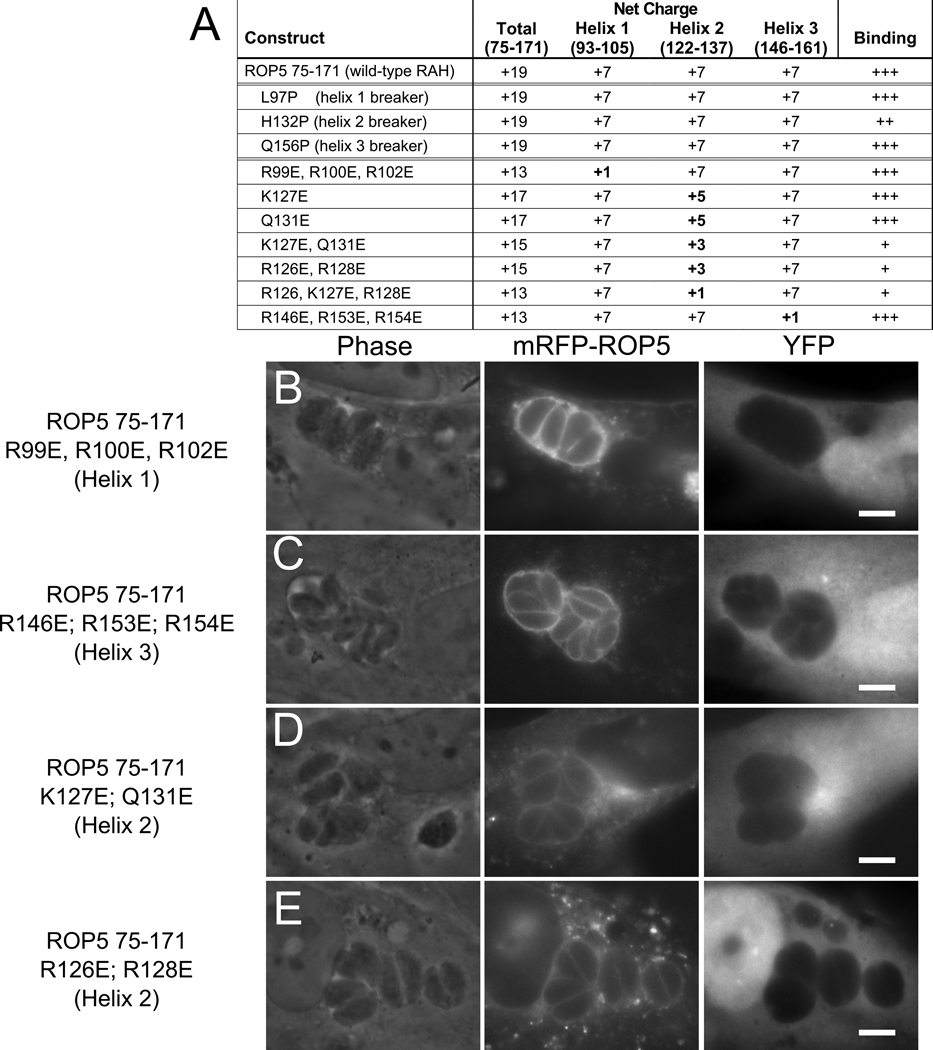
Figure 7. The polar surface of RAH helix 2 encodes PVM-specific binding.
(A) Overview of mutant constructs. The net charge of each construct and a summary of the quality of PVM association are listed. Mutants of mRFP-ROP5RAH were expressed in infected fibroblasts concurrently with soluble YFP as a cellular morphology marker. (B) Protein containing mutations in the first helix (R99E, R100E, R102E) that neutralize the positive charge of the polar face displays wild-type specificity for the PVM. (C) Similar mutations in the third helix (R146E, R153E, R154E) also have no effect on PVM specificity. Mutations in the second helix (D) K127E, Q131E or (E) R126E, R128E, that halve the net charge, attenuate the RAH PVM specificity.