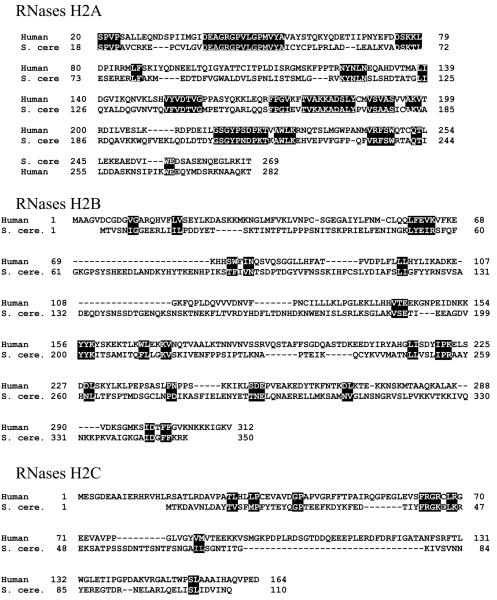
Figure 4.
(A) Comparison of amino acid sequences of human and S. cerevisiae RNase H2 subunits. Alignments are based on the consensus alignment of RNase H2 subunits from Crow et al. Reverse letters highlight homologies between the subunits. For RNases H2B and RNases H2C, a more generous highlighting is used and even then it is clear there is very little conservation between the human and yeast B and C subunits. (B) Schematic representation of the three components of human RNase H2. On top of each subunit are shown AGS-related mutations, and on the bottom the amino acids involved in catalysis, in RNASEH2A, and the PIP (PCNA Interacting Peptide) site of RNASEH2B.