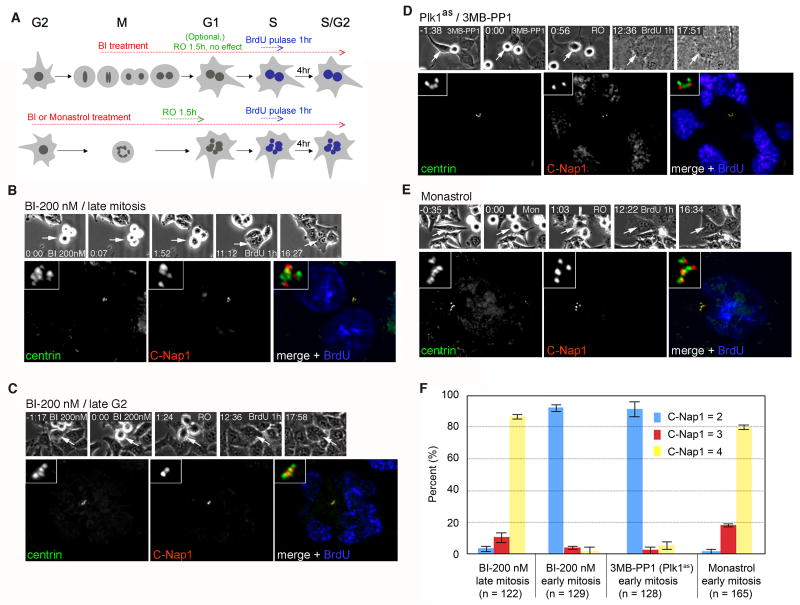
Figure 4. Plk1 acts during early mitosis to promote centriole disengagement.
(A) Experimental scheme. Asynchronously proliferating cells were filmed during a 3 hour treatment with the Plk1 inhibitor BI-2536 (BI) or the Eg5 inhibitor monastrol as a control. We note that Plk1 inactivation in late G2 or prophase activates the spindle assembly checkpoint and arrests cells in prometaphase. In contrast, late mitotic Plk1 inactivation in does not block anaphase onset, but instead inhibits cytokinesis. To allow analysis of centriole duplication potential under both treatment regimens, cells in the former population were induced to exit mitosis using the Cdk1-selective inhibitor RO-3306 (RO). Cells transiting through S phase were marked by BrdU pulse-labeling and analyzed as in Fig. 3. (B) A hESPL1flox/Δ cell treated with BI during late M phase (200 nM) exhibits complete centriole disengagement (4 C-Nap1 foci) and duplication (8 centrin foci). (C) A hESPL1flox/Δ cell treated with BI during late G2 (200 nM), showing no disengagement (2 C-Nap1 foci) and no duplication (4 centrin foci). (D) A Plk1as cell (RPE1, retinal pigment epithelial human cells) treated with 3MB-PP1 (10 mM) during late G2, showing no disengagement (2 C-Nap1 foci) and no duplication (4 centrin foci). (E) A hESPL1flox/Δ cell treated with monastrol (50 μM) during late G2 exhibits complete disengagement (4 C-Nap1 foci) and duplication (8 centrin foci, one of which lies in a different focal plane (not shown)). (F) Quantification of results in B-E. Error bars indicate standard deviations from three independent experiments.