Abstract
Background
Neuroblastoma is the most common extracranial pediatric solid cancer. Lung metastasis is rarely detected in children with newly diagnosed neuroblastoma. We aimed to describe the incidence, clinical characteristics, and outcome of patients with lung metastasis at initial diagnosis using a large international database.
Procedure
The subset of patients from the International Neuroblastoma Risk Group database with INSS stage 4 neuroblastoma and known data regarding lung metastasis at diagnosis was selected for analysis. Clinical and biological characteristics were compared between patients with and without lung metastasis. Survival for patients with and without lung metastasis was estimated by Kaplan-Meier methods. Cox proportional hazards methods were used to determine the independent prognostic value of lung metastasis at diagnosis.
Results
Of the 2,808 patients with INSS stage 4 neuroblastoma diagnosed between 1990 and 2002, 100 patients (3.6%) were reported to have lung metastasis at diagnosis. Lung metastasis was more common among patients with MYCN amplified tumors, adrenal primary tumors, or elevated lactate dehydrogenase (LDH) levels (p < 0.02 in each case). Five-year overall survival ± standard error for patients with lung metastasis was 34.5% ± 6.8% compared to 44.7% ± 1.3% for patients without lung metastasis (p=0.0002). However, in multivariable analysis, the presence of lung metastasis was not independently predictive of outcome.
Conclusions
Lung metastasis at initial diagnosis of neuroblastoma is associated with MYCN amplification and elevated LDH levels. Although lung metastasis at diagnosis was not independently predictive of outcome in this analysis, it remains a useful prognostic marker of unfavorable outcome.
Keywords: Neuroblastoma, Lung Metastases, Pulmonary, MYCN
Introduction
The most common sites of metastasis in neuroblastoma are the bone and bone marrow, with involvement of these sites found in the majority of children with newly diagnosed metastatic neuroblastoma [1]. In contrast, lung metastasis is a distinctly uncommon finding in children presenting with metastatic neuroblastoma. One group reviewed their single institution experience and found that 3 out of 104 patients with neuroblastoma of any stage had chest radiograph findings consistent with lung metastases at initial diagnosis [2]. Of the 567 patients with stage 4 neuroblastoma treated on Children's Cancer Group protocols 3881 and 3891, 21 patients had lung metastases reported at initial diagnosis (incidence of 3.7%) [1,3]. This group was characterized by a high incidence of MYCN amplification and a poor outcome. Data from the European Neuroblastoma Study Group included 5 patients with radiographically identified lung metastases at presentation out of 746 patients with stage 4 disease (incidence of 0.7%) [4].
The International Neuroblastoma Risk Group (INRG) task force is a collaborative effort between groups in Europe, North America, Australia, New Zealand, and Japan. The INRG database currently includes data on over 8,000 patients enrolled on a neuroblastoma study from January 1990 to December 2002. The large size and extended follow-up of this contemporary dataset provide an opportunity to formally investigate unusual presentations of neuroblastoma. Using this large database of patients, we aimed to further describe the incidence, clinical characteristics, and outcome of neuroblastoma with lung metastasis at initial diagnosis.
Methods
Patients
The INRG database includes patients ≤ 21 years of age with pathologically confirmed neuroblastoma diagnosed between January 1, 1990 and December 31, 2002. All patients were enrolled on a neuroblastoma study within the following INRG countries or cooperative groups: France; Germany; Japan; Italy; Spain; United Kingdom; Children's Oncology Group; and SIOP LNESG1 study. Members of the INRG are listed in the Appendix. Of the 8,800 unique patients included in the INRG database, the subset of patients with international neuroblastoma staging system (INSS) stage 4 disease and known data regarding lung metastasis was selected for this analysis [5]. The INRG database does not include detailed data on the extent of chest imaging performed at initial diagnosis. Given the international nature of the database, central radiographic review to confirm lung metastasis was not possible.
Statistical Methods
Differences between patients with and without lung metastasis were evaluated using the Fisher's exact test. Clinical variables of interest included age, site of primary tumor, and involvement of other metastatic sites. Biological variables of interest included lactate dehydrogenase level (LDH) at diagnosis, ferritin level at diagnosis, Shimada histology classification, MYCN amplification, loss of heterozygosity at 1p, 11q aberration, and gain of 17q [6-11]. For LDH and ferritin, the median values from the entire INRG cohort were 580 units/L and 96 ng/mL, respectively. These thresholds were used to dichotomize patients into two categories: elevated (at or above the median) and not elevated (below the median). Similarly, age was treated as a dichotomous variable (less than 18 months or greater than/equal to 18 months at diagnosis) [12].
Estimated event-free and overall survival were determined using Kaplan-Meier methods, with time to event (relapse, progression, secondary malignancy, death) calculated from the date of neuroblastoma diagnosis [13]. Survival estimates are presented for the 5-year timepoint as the estimate ± standard error, with the standard errors calculated using the methods of Peto [14]. Differences in survival between patients with and without lung metastasis were evaluated using the log-rank test. A Cox proportional model was built to determine the hazard ratio for event-free survival (EFS) for patients with lung metastasis compared to patients without lung metastasis. In addition to the presence or absence of lung metastases at initial diagnosis, covariates evaluated in this model included those clinical and biological features that were prognostic of outcome in univariate analysis. For all analyses, a p-value < 0.05 was considered statistically significant. All statistical analyses were performed using SAS, version 9.
Results
The analytic cohort for this report included 2,808 unique patients from the INRG database with INSS stage 4 neuroblastoma and with known data regarding lung metastasis. Of these, 100 patients were reported to have lung metastasis at initial diagnosis, giving an incidence of 3.6%. Since all children with neuroblastoma do not undergo routine chest imaging, the incidence of lung metastasis at initial diagnosis was also determined within the group of patients with thoracic primary tumors, as nearly all of these patients would be expected to undergo chest imaging. Within the group of 274 patients who had thoracic primary tumors, only 5 patients had lung metastases at diagnosis, yielding an incidence of 1.8%.
The characteristics of the 2,808 patients with stage 4 neuroblastoma, including 100 with lung metastasis at initial diagnosis, are shown in Table I. Patients with lung metastasis tended to be slightly younger than patients without lung metastasis, but this difference was not statistically significant. Patients with lung metastasis were more likely to have adrenal primary tumors than patients without lung metastasis (p = 0.017). Patients with lung metastasis had a higher incidence of concomitant metastasis to the central nervous system (CNS) or liver compared to patients without lung metastasis in whom these metastatic sites were relatively uncommon (p < 0.01 for CNS or liver metastasis). More patients with lung metastasis had elevated LDH levels compared to patients without lung metastasis (p = 0.011). Fifty-four percent of patients with lung metastasis had MYCN amplified tumors, compared with only 29% of patients without lung metastasis (p < 0.001). Though the number of patients with available data is small, the presence of an 11q aberration appeared to be less frequent in patients with lung metastasis compared to patients without lung metastasis (p = 0.086). Other clinical and biological features did not differ significantly between patients with and without lung metastasis, including serum ferritin levels, presence of skin metastasis, and gain of 17q (data not shown).
Table I.
Characteristics of patients with stage 4 neuroblastoma in the INRG database with and without lung metastasis at initial diagnosis.
| Lung Metastasis n = 100 |
No Lung Metastasis n = 2,708 |
|
|---|---|---|
| Median Age at Diagnosis (days) | 670 | 914 |
| Age at Diagnosis ≥ 18 months | 61 / 100 (61%) | 1864 / 2708 (68.8%) |
| Adrenal Primary Site* | 69 / 99 (69.7%) | 1544 / 2691 (57.4%) |
| Thoracic Primary Site | 5 / 99 (5.1%) | 269 / 2693 (10.0%) |
| Bone Marrow Metastasis | 59 / 100 (59%) | 1692 / 2707 (62.5%) |
| Bone Metastasis | 53 / 100 (53%) | 1351 / 2705 (49.9%) |
| Liver Metastasis* | 33 / 100 (33%) | 388 / 2708 (14.3%) |
| Lymph Node Metastasis | 21 / 100 (21%) | 662 / 2698 (24.5%) |
| CNS Metastasis* | 9 / 100 (9%) | 72 / 2708 (2.7%) |
| Elevated LDH* | 55 / 67 (82.1%) | 1031 / 1538 (67.0%) |
| Unfavorable Shimada Histology | 31 / 39 (79.5%) | 853 / 1196 (71.3%) |
| MYCN Amplification* | 42 / 78 (53.9%) | 638 / 2192 (29.1%) |
| Loss of Heterozygosity at 1p | 12 / 25 (48.0%) | 214 / 593 (36.1%) |
| 11q Aberration | 2 / 13 (15.4%) | 151 / 372 (40.6%) |
p< 0.05 for the difference between patients with and without lung metastases.
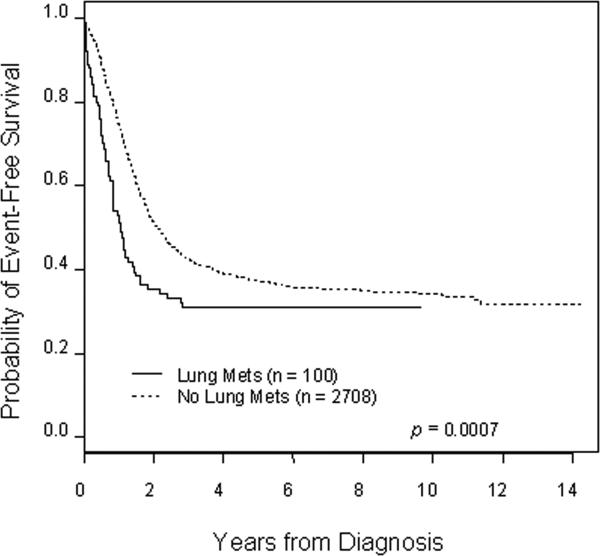
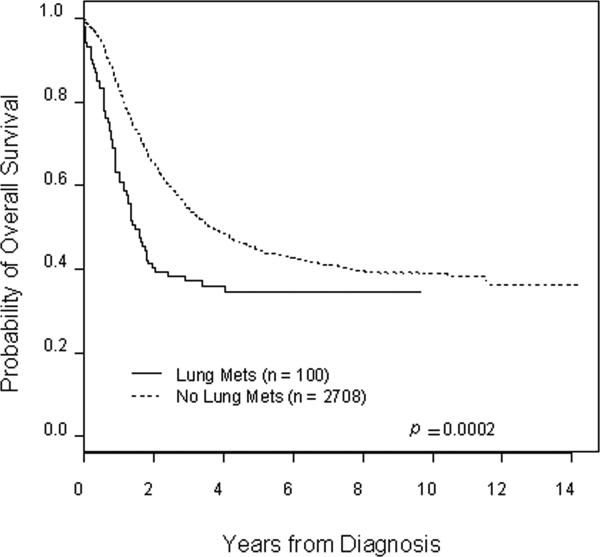
Patients with lung metastasis at initial diagnosis had an inferior outcome compared to patients without lung metastasis and this difference is statistically significant for both event-free (Figure 1) and overall survival (Figure 2) (p < 0.001 in each case). Patients with lung metastasis appeared to be at increased risk for early events and early death compared to patients without lung metastasis, with hazard ratios of 1.52 (95% confidence interval 1.19−1.93) and 1.62 (95% confidence interval 1.26−2.08) for event-free and overall survival, respectively. Estimated event-free survival five years from diagnosis for patients with lung metastasis was 30.9% ± 7.1% compared to 37.2% ± 1.2% for patients without lung metastasis. Five-year overall survival for patients with lung metastasis was 34.5% ± 6.8% compared to 44.7% ± 1.3% for patients without lung metastasis. No deaths occurred in patients with lung metastasis beyond five years from initial diagnosis.
Figure 1.
Kaplan-Meier estimated event-free survival for patients with and without lung metastasis at initial diagnosis of INSS stage 4 neuroblastoma. The hazard ratio for increased risk of event with lung metastasis is 1.52 (95%) confidence interval of 1.19−1.93).
Figure 2.
Kaplan-Meier estimated overall survival for patients with and without lung metastasis at initial diagnosis of INSS stage 4 neuroblastoma. The hazard ratio for increased risk of death with lung metastasis is 1.62 (95% confidence interval of 1.26−2.08).
In order to determine the prognostic impact of lung metastasis independent of potential confounders, a Cox proportional hazards model for event-free survival was constructed. The model tested factors found prognostic of outcome in univariate analyses: adrenal primary tumor site, elevated LDH level, MYCN amplification, and age ≥ 18 months. Presence of an 11q aberration was not tested in this model due to small patient numbers. After controlling for statistically significant factors of MYCN amplification, age, and LDH level, the presence of lung metastases was not found to be independently prognostic of worse EFS.
Discussion
This analysis provides an evaluation of lung metastasis at initial diagnosis in a large group of patients with metastatic neuroblastoma. The results indicate that patients presenting with lung metastasis have an aggressive phenotype that is characterized by MYCN amplified tumors, LDH elevation, metastasis to the CNS, and a poor outcome. In addition, this international collaborative effort provides a model with which to study rare events in pediatric oncology.
These results confirm the previously reported low incidence of lung metastasis at initial diagnosis in patients with stage 4 neuroblastoma [1,3,4]. This finding supports current clinical practice, which does not include routine chest imaging for patients with non-thoracic primary tumors. The incidence of 3.6% may be an underestimate since not all patients underwent detailed chest imaging. The INRG database does not include data on imaging studies performed at initial diagnosis. However, one may speculate that most of these patients had a chest radiograph obtained to confirm central venous catheter position early in the course of therapy. Of note, the incidence of lung metastasis in patients with thoracic primary tumors was only 1.8%. Since most of these patients would be expected to receive detailed chest imaging, this estimate is likely close to the true incidence of lung metastasis in patients with thoracic neuroblastoma. This result suggests that the incidence of lung metastasis may be lower in patients with thoracic neuroblastoma compared to the overall incidence of 3.6%. Given that lung metastasis appears to be associated with unfavorable biological features, this result is consistent with previous findings indicating that thoracic tumors have more favorable biological features [15].
The low incidence of lung metastasis in neuroblastoma is somewhat unique, as the lung is the most common site of metastasis in most other pediatric extra-cranial solid cancers. Among patients presenting with initially metastatic disease, approximately 50% of patients with Ewing sarcoma and 80% of patients with osteosarcoma or Wilms tumor have lung metastases [16-18]. The etiology for this low incidence of lung metastasis in neuroblastoma remains unclear, particularly since neuroblastoma exhibits hematogenous dissemination to other sites. An early analysis of the metastatic pattern in neuroblastoma concluded that neuroblastoma metastasis does not passively follow the pattern of blood flow [19]. Several case reports describe patients with neuroblastoma invading the inferior vena cava at diagnosis, only a subset of whom had detectable lung metastasis [20-22]. One may speculate that specific features of the pulmonary microenvironment are hostile to neuroblastoma growth.
Patients with lung metastasis at initial diagnosis appear to have particularly aggressive tumors that counteract the factors that typically impede the establishment of pulmonary metastases in neuroblastoma. These patients have higher LDH levels than patients without lung metastases. This finding may reflect greater tumor cell turnover in these patients or an increase in LDH due to the presence of injured lung tissue. These patients also have tumors which are more likely to be MYCN amplified compared to patients without lung metastasis. Patients with lung metastasis at initial diagnosis also appear to have a higher incidence of metastasis to the CNS, another unusual site of dissemination at initial presentation.[1] These patients follow a more aggressive clinical course, with increased risk for early disease progression and early death. However, the results of the Cox proportional hazards model indicate that lung metastasis at initial diagnosis is not an independent predictor of unfavorable outcome.
The size of the INRG database allowed for a more thorough evaluation of the clinical characteristics and prognosis of patients with lung metastasis than previously possible. The main limitation of this study was the inability to confirm reports of lung metastasis through central radiology review. Some reports of lung metastasis may have represented pleural involvement or direct extension from thoracic primary tumors. The INRG database does not include details on whether reported lung metastases were pathologically proven. Despite these concerns, the results appear consistent with previous reports in which central radiographic review was performed.
In conclusion, patients with stage 4 neuroblastoma only rarely have detectable lung metastasis at initial diagnosis. Lung metastasis at diagnosis is a marker of unfavorable outcome. Future investigations should focus on the factors in the pulmonary microenvironment that impede the development of metastasis in the lung. Such investigations may provide further insight into the factors required for neuroblastoma growth and thereby provide new targets for therapeutic intervention.
Supplementary Material
Research Support
The International Neuroblastoma Risk Group Task Force is supported by The William Guy Forbeck Research Foundation and the Little Heroes Pediatric Cancer Research Foundation.
Footnotes
Disclaimers: None
Previous Presentations: Presented in part at the 2007 American Society of Pediatric Hematology/Oncology Annual Meeting
References
- 1.DuBois SG, Kalika Y, Lukens JN, et al. Metastatic sites in stage IV and IVS neuroblastoma correlate with age, tumor biology, and survival. J Pediatr Hematol Oncol. 1999;21:181–189. doi: 10.1097/00043426-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 2.Towbin R, Gruppo RA. Pulmonary metastases in neuroblastoma. AJR Am J Roentgenol. 1982;138:75–78. doi: 10.2214/ajr.138.1.75. [DOI] [PubMed] [Google Scholar]
- 3.Kammen BF, Matthay KK, Pacharn P, et al. Pulmonary metastases at diagnosis of neuroblastoma in pediatric patients: CT findings and prognosis. AJR Am J Roentgenol. 2001;176:755–759. doi: 10.2214/ajr.176.3.1760755. [DOI] [PubMed] [Google Scholar]
- 4.Cowie F, Corbett R, Pinkerton CR. Lung involvement in neuroblastoma: incidence and characteristics. Med Pediatr Oncol. 1997;28:429–432. doi: 10.1002/(sici)1096-911x(199706)28:6<429::aid-mpo7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Brodeur GM, Seeger RC, Barrett A, et al. International criteria for diagnosis, staging, and response to treatment in patients with neuroblastoma. J Clin Oncol. 1988;6:1874–1881. doi: 10.1200/JCO.1988.6.12.1874. [DOI] [PubMed] [Google Scholar]
- 6.Attiyeh EF, London WB, Mosse YP, et al. Chromosome 1p and 11q deletions and outcome in neuroblastoma. N Engl J Med. 2005;353:2243–2253. doi: 10.1056/NEJMoa052399. [DOI] [PubMed] [Google Scholar]
- 7.Bown N, Cotterill S, Lastowska M, et al. Gain of chromosome arm 17q and adverse outcome in patients with neuroblastoma. N Engl J Med. 1999;340:1954–1961. doi: 10.1056/NEJM199906243402504. [DOI] [PubMed] [Google Scholar]
- 8.Hann HW, Evans AE, Siegel SE, et al. Prognostic importance of serum ferritin in patients with Stages III and IV neuroblastoma: the Childrens Cancer Study Group experience. Cancer Res. 1985;45:2843–2848. [PubMed] [Google Scholar]
- 9.Seeger RC, Brodeur GM, Sather H, et al. Association of multiple copies of the N-myc oncogene with rapid progression of neuroblastomas. N Engl J Med. 1985;313:1111–1116. doi: 10.1056/NEJM198510313131802. [DOI] [PubMed] [Google Scholar]
- 10.Shimada H, Chatten J, Newton WA, Jr., et al. Histopathologic prognostic factors in neuroblastic tumors: definition of subtypes of ganglioneuroblastoma and an age-linked classification of neuroblastomas. J Natl Cancer Inst. 1984;73:405–416. doi: 10.1093/jnci/73.2.405. [DOI] [PubMed] [Google Scholar]
- 11.Shuster JJ, McWilliams NB, Castleberry R, et al. Serum lactate dehydrogenase in childhood neuroblastoma. A Pediatric Oncology Group recursive partitioning study. Am J Clin Oncol. 1992;15:295–303. doi: 10.1097/00000421-199208000-00004. [DOI] [PubMed] [Google Scholar]
- 12.London WB, Castleberry RP, Matthay KK, et al. Evidence for an age cutoff greater than 365 days for neuroblastoma risk group stratification in the Children's Oncology Group. J Clin Oncol. 2005;23:6459–6465. doi: 10.1200/JCO.2005.05.571. [DOI] [PubMed] [Google Scholar]
- 13.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J Royal Stat Soc Series A. 1972;135:185–198. [Google Scholar]
- 15.Morris JA, Shcochat SJ, Smith EI, et al. Biological variables in thoracic neuroblastoma: a Pediatric Oncology Group study. J Pediatr Surg. 1995;30:296–302. doi: 10.1016/0022-3468(95)90577-4. discussion 302−293. [DOI] [PubMed] [Google Scholar]
- 16.Breslow NE, Churchill G, Nesmith B, et al. Clinicopathologic features and prognosis for Wilms’ tumor patients with metastases at diagnosis. Cancer. 1986;58:2501–2511. doi: 10.1002/1097-0142(19861201)58:11<2501::aid-cncr2820581125>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 17.Kager L, Zoubek A, Potschger U, et al. Primary metastatic osteosarcoma: presentation and outcome of patients treated on neoadjuvant Cooperative Osteosarcoma Study Group protocols. J Clin Oncol. 2003;21:2011–2018. doi: 10.1200/JCO.2003.08.132. [DOI] [PubMed] [Google Scholar]
- 18.Paulussen M, Ahrens S, Burdach S, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. European Intergroup Cooperative Ewing Sarcoma Studies. Ann Oncol. 1998;9:275–281. doi: 10.1023/a:1008208511815. [DOI] [PubMed] [Google Scholar]
- 19.de la Monte SM, Moore GW, Hutchins GM. Nonrandom distribution of metastases in neuroblastic tumors. Cancer. 1983;52:915–925. doi: 10.1002/1097-0142(19830901)52:5<915::aid-cncr2820520529>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 20.Bagatell R, Morgan E, Cosentino C, Whitesell L. Two cases of pediatric neuroblastoma with tumor thrombus in the inferior vena cava. J Pediatr Hematol Oncol. 2002;24:397–400. doi: 10.1097/00043426-200206000-00015. [DOI] [PubMed] [Google Scholar]
- 21.Custodio CM, Semelka RC, Balci NC, et al. Adrenal neuroblastoma in an adult with tumor thrombus in the inferior vena cava. J Magn Reson Imaging. 1999;9:621–623. doi: 10.1002/(sici)1522-2586(199904)9:4<621::aid-jmri17>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Nagda SN, Lo SS, Melian E, et al. Unusual thoracic problems in patients with malignancies: case 1. Neuroblastoma presenting with intracardiac tumor thrombus. J Clin Oncol. 2005;23:2856–2857. doi: 10.1200/JCO.2005.01.115. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.