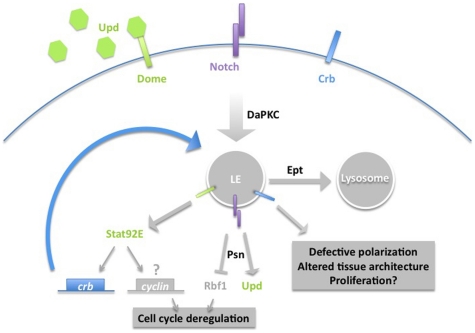
Figure 7. A hypothetical model of the cell-autonomous effects of ept loss.
This model incorporates data from this paper and an accompanying study on the link between ept, DaPKC, Notch, Crb, Psn and Rbf1. The Erupted protein (Ept) is required to traffic Crb (blue), Notch (purple), and Dome (green) into the lysosome. DaPKC may regulate an upstream step in this process by promoting internalization of Notch and Crb. Loss of Ept causes Notch, Crb, and Dome (and perhaps other unidentified receptors) to accumulate in the endosome, from which they drive downstream effects. Stat92E likely has many nuclear targets in ept mutant cells, including crb. Given the links between mammalian Stats and G1 cyclin expression, Stat92E may have the potential to affect expression of a cyclin as well. Presenilin (Psn) acts upstream of Rbf1, perhaps via its role in activating Notch; a similar role for Psn upstream of the Notch-target Upd is untested but would follow logically from the available data. The excess Crb expressed in ept mutant cells is predicted to cycle back into the late endosome (LE), leading to very high levels of vesicular Crb. Overexpressed Crb is known to disrupt tissue architecture (e.g. [30]) and may to do the same in the context of ept tumors.