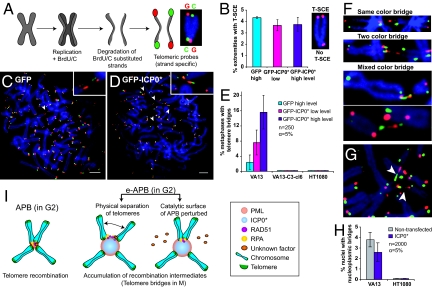
Fig. 4.
Enlargement of APBs perturbs the resolution of recombination intermediates between telomeres located on different chromosomes. (A) Chromosome-oriented (CO-) FISH procedure. (B) The level of telomere sister chromatid exchange (T-SCE) remains unperturbed in VA13 cells upon expression of ICP0* as determined by CO-FISH using a probe against the G-rich strand (red) (See also Fig. S5). n = 40–42 metaphases. Error bars, SEM. (C and D) CO-FISH analysis revealing telomere recombination intermediates or telomere bridges (arrowheads) whose incidence increases upon transfection of VA13 cells with ICP0*. (Scale bars, 5 μm.) (E) Percentage of metaphase spreads with telomere bridges in VA13, VA13-C3-cl6 and HT1080 cells. Error bars represent the confidence interval. (F) Representative images for different categories of telomere bridges based on the pattern of parental G-rich (red) and C-rich (green) telomeric DNA strands. (G) Two sister chromatids forming two independent telomere bridges (arrowheads) with two different chromosomes. (H) Increased incidence of telomere bridges is not accompanied by an increase in nucleoplasmic bridges. Error bars represent the confidence interval. (I) We propose two, non-exclusive, models of how ICP0* interferes with the recombination of telomeres in enlarged (e-)APBs: ICP0* may prematurely promote the physical separation of telomeres or perturb the catalytic surface of APBs perhaps by inducing the relocation of recombination factors from APBs into the nucleoplasm.