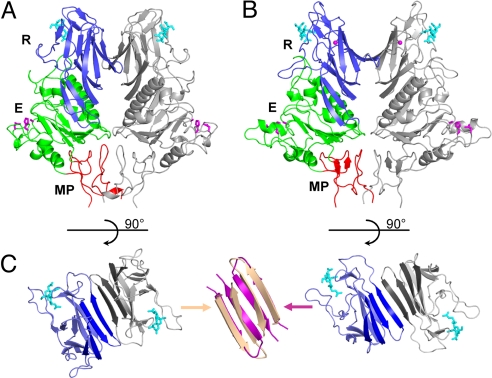
Fig. 1.
Overall structure of ToV HE and comparison to CoV HE. (A) Ribbon representation of the PToV HE dimer. One HE monomer is colored gray, and the other is colored by domain: lectin domain (R: 143–284, blue) with bound Neu4,5,9Ac3α2Me (cyan sticks), esterase domain (E: 38–142 and 285–350, green) with Ser-His-Asp active site triad (magenta sticks), and membrane-proximal domain (MP: 25–37 and 351–386, red). The overall structure of the BToV HE dimer is very similar. (B) Ribbon representation of the BCoV HE dimer colored as in A. (C) Top views of the R domains in the PToV (Left) and the BCoV HE dimer (Right). A continuous 8-stranded β-sheet (emphasized by darker coloring) is formed across the dimer interface in BCoV HE, but not in PToV HE. Superposition (Center) of β-strands at the interface shows that strands in PToV HE (wheat) are more twisted than in BCoV HE (magenta), which prevents formation of a continuous β-sheet.