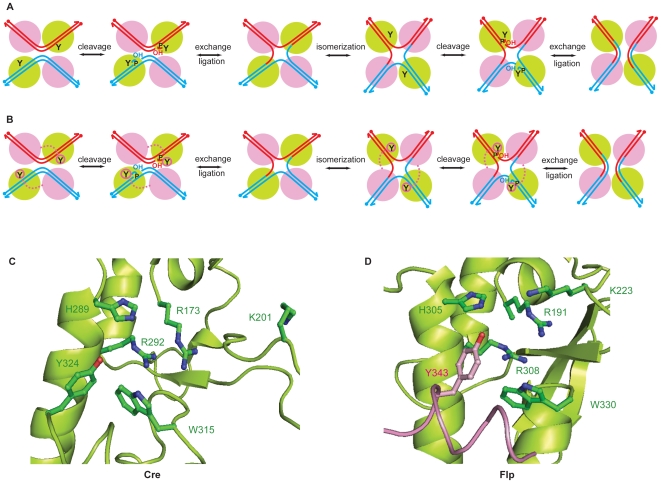
Figure 1. Reaction pathways and active site configurations for tyrosine site-specific recombination promoted by Cre and Flp.
A, B. Recombination by Flp and Cre proceeds through two steps of single strand exchanges via a Holliday junction intermediate. During strand cleavage, the pair of recombinase monomers in green activates the scissile phosphodiester bonds in cis for nucleophilic attack by the catalytic tyrosine. The pair of Flp monomers in magenta donates the tyrosine nucleophile in trans. The corresponding Cre monomers are thought to allosterically confer chemical competence on the green monomers that perform strand cleavage in cis. B, C. Cross-sections of the Cre and Flp active sites containing the catalytic pentad and the tyrosine nucleophile are shown [5], [6]. In Flp, this tyrosine (shown in magenta) is donated by the neighboring Flp monomer.