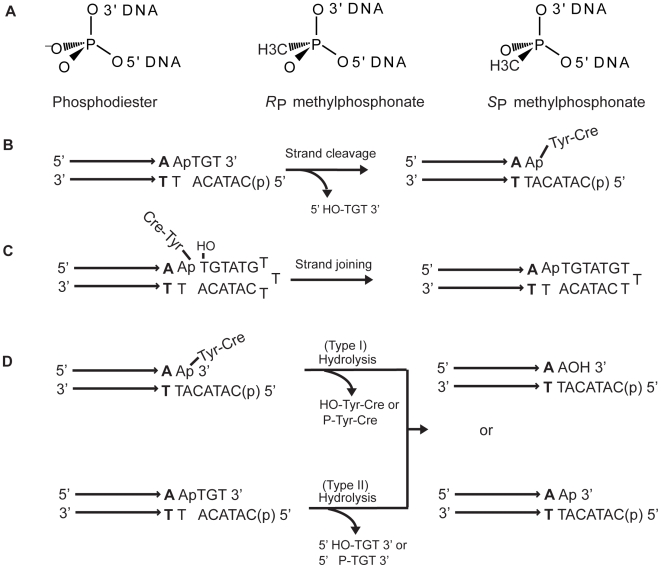
Figure 2. Half-site substrates for Cre reactions containing a phosphodiester or methylphosphonate at the scissile position.
A. Substitution of a nonbridging oxygen atom of the achiral phosphodiester position in a DNA chain will generate either the R P or S P stereoisomer of methylphosphonate. B. In the schematic representation of a half-site substrate, the horizontal arrows represent a Cre binding element with its terminal bp abutting the spacer shown in bold. The scissile phosphodiester is indicated as ‘p’. Strand cleavage in a half-site traps the tyrosyl intermediate, as the trinucleotide product (5′HO-TGT3′) diffuses away. The 5′ end of the bottom strand is blocked by phosphorylation (denoted by ‘p’ in parentheses) from acting as the nucleophile in a joining reaction. C. A half-site designed to probe strand joining contains a longer bottom strand ending in a 5′-hydroxyl group. This hydroxyl could be positioned for nucleophilic attack in the cleaved intermediate by the single stranded region looping back as diagrammed. D. Possible products from hydrolysis of the tyrosyl intermediate resulting from strand cleavage (type I) or direct hydrolysis of the scissile phosphodiester in DNA (type II) are indicated. The presence of a hydroxyl or a phosphate group at the 3′ end in the hydrolytic product is determined by the line of attack of the water nucleophile.