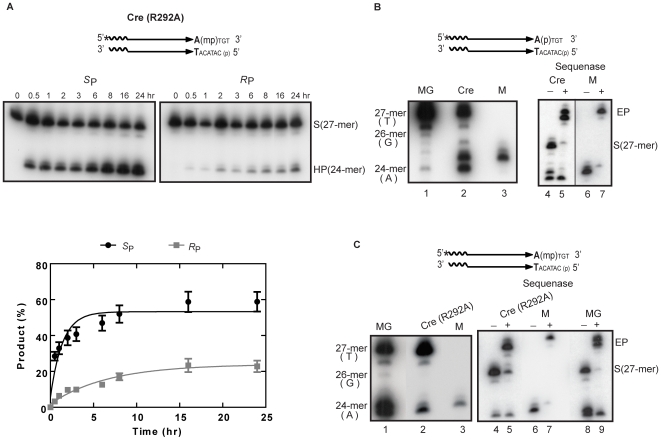
Figure 6. Stereochemical preference of Cre(R292A) in the MeP-half-site reaction and characterization of the hydrolysis products from P- and MeP-half-site substrates.
A. The reactions of stereochemically pure S P and R P forms of the MeP-half-site with Cre(R292A) were analyzed by denaturing PAGE. B. The closely migrating hydrolysis products from the P-half-site (see Figure 3; lane 2 denotes a 24 hr reaction with Cre) were characterized for the chemical nature their 3′ ends. ‘MG’ refers to a Maxam-Gilbert sequencing ladder obtained by chemical modification at C+T positions followed by strand scission. The sizes of the oligonucleotides and the 3′-terminal bases they harbor are indicated against the rungs of the sequencing ladder. ‘M’ stands for a synthetic oligonucleotide marker (24-mer) with the expected sequence of the hydrolysis product and harboring a 3′-OH end. ‘EP’ refers to the extension product formed in a sequenase reaction. C. The hydrolysis product from a 6 hr Cre(R292A)/MeP-half-site reaction (lane 2) was subjected to similar analyses as in B. The marker oligonucleotide M was the same as that shown in B, harboring a hydroxyl group at the 3′ end. Strand breakage at the MeP position in the sequencing reaction produced a strong doublet (lanes 1 and 8). The top band of this doublet was extended in the elongation reaction, whereas the bottom band was not (compare lanes 8 and 9). In the sequenase reactions shown in B and C, the band marked S (27-mer) refers to the labeled top strands, carrying a 3′-hydroxyl end, of the P- or MeP-half-site. As expected, these oligonucleotides served as primers for sequenase.