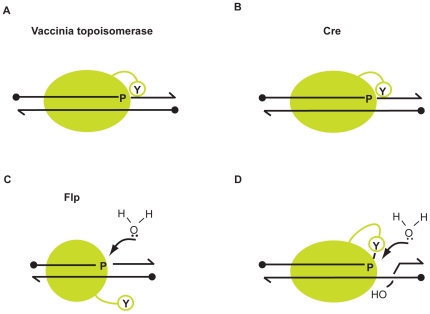
Figure 7. Mechanisms for the protection of the transesterification steps of DNA relaxation and site-specific recombination from hydrolysis.
A, B. The strand cleavage steps of DNA relaxation by topoisomerase and recombination by Cre are protected by the temporal coupling between protein-DNA association and engagement of the scissile phosphate by the cis-acting active site. C. Binding of a Flp monomer to DNA activates the scissile phosphate but full engagement of the active site must await the binding of a second Flp monomer and donation of the active site tyrosine. Hydrolysis of the phosphodiester bond becomes a potential risk, which is minimized through active site electrostatics. D. During topoisomerase action, the cleaved tyrosyl intermediate is a potential target for attack by water during the strand rotation step that precedes ligation. The danger of hydrolysis at this stage is avoided through phosphate electrostatics.