Figure 6.
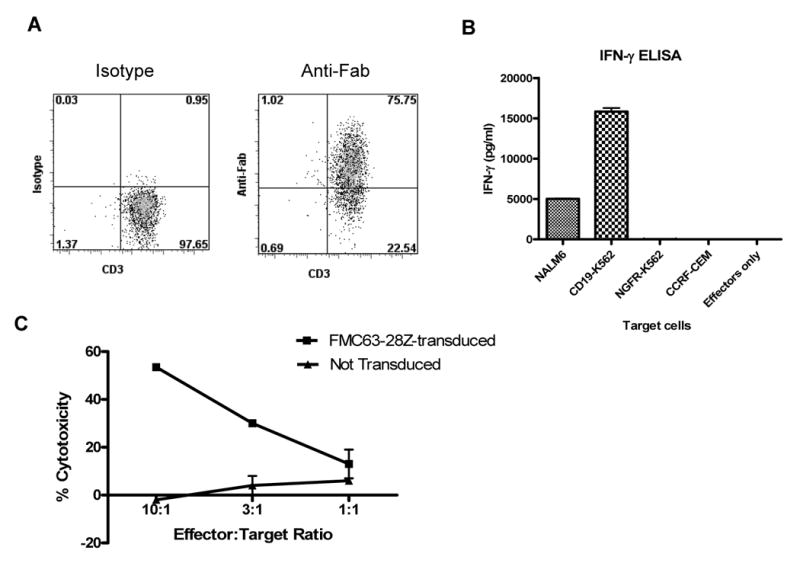
Large numbers of functional anti-CD19 T cells can be generated from the blood of CLL patients. (A) T cells from a patient with CLL that had received fludarabine plus rituximab were transduced with FMC63-28Z and induced to proliferate in a REP. On day 10 after initiation of the REP 75% of the T cells expressed the FMC63-28Z CAR as measured by staining with anti-Fab antibodies. Cells from six different CLL patients were transduced in a similar manner, and the percentage of T cells from each of these patients that expressed the FMC63-28Z CAR ten days after the initiation of a REP is reported in Table 3. (B) Ten days after initiation of a REP, cells from the same culture used for the experiment reported in (A) produced IFNγ upon stimulation with the CD19-expressing cell lines NALM6 and CD19-K562 but not the CD19-negative cell lines NGFR-K562 and CCRF-CEM. These results are representative of six experiments in which cells from six different CLL patients were used. (C) FMC63-28Z-transduced T cells from a CLL patient specifically killed autologous CLL cells while nontransduced T cells from the same patient exhibited minimal cytotoxicity 12 days after initiation of a REP. Cytotoxicity is reported as the mean +/- the SEM of duplicate determinations. These results are representative of two experiments in which cells from two different patients were used.
