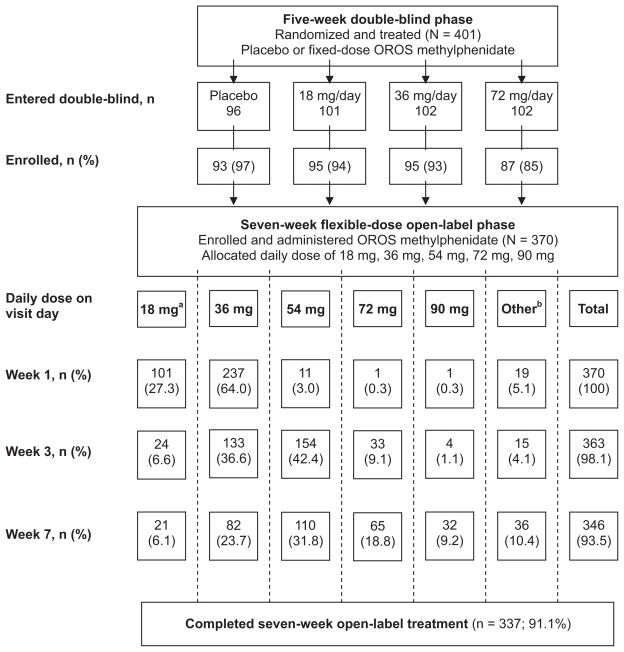
Figure 1.
Flow of patients from the double-blind phase through the open-label phase of the trial.
Notes: aPatients at German centers began with 18 mg/day; bOther includes patients who took no drug on the visit day or did not have a visit during the time interval (week 1 [1–13 days], week 3 [14–31 days], week 7 [≥32 days]).
Abbreviation: OROS, osmotic release oral system.