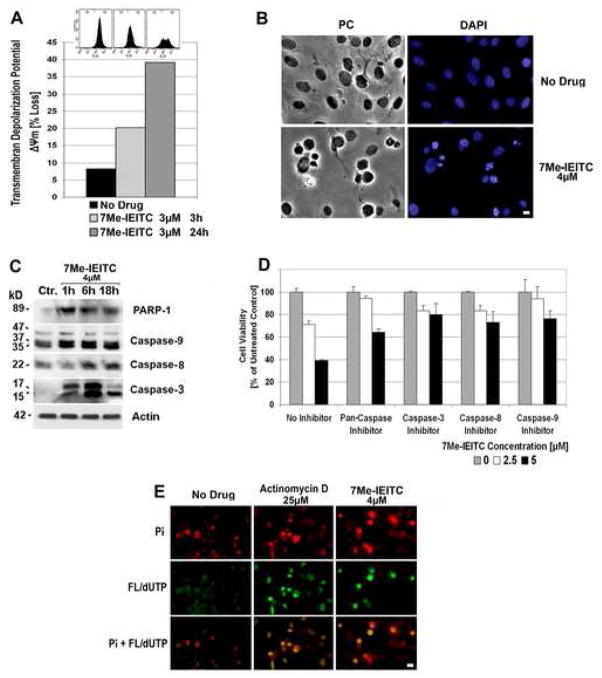
Figure 3. 7Me-IEITC causes apoptosis in platinum-resistant SKOV-3 ovarian cancer cells.
Panel A: Membrane depolarization analysis after 7Me-IEITC treatment. SKOV-3 cells were treated for 3 or 24hrs with 3μM 7Me-IEITC, fixed and stained with DiOC6(3) as described (Materials and Methods). Fluorescence of the single cell population was measured by flow cytometry (excitation at 488nm, emission at 520nm; insert at top of panel B) and the transmembrane depolarization potential of the single cell populations plotted (bar chart). Ten thousand cells were analyzed in each sample.
Panel B: Morphological changes after 7Me-IEITC treatment. SKOV-3 cells were treated for 24hrs with 4μM 7Me-IEITC, fixed and stained with 4′-6-Diamidino-2-Phenylindole (DAPI) as described (Materials and Methods) before mounting. Microscopy was carried out (Nikon Eclipse TE2000-E inverted microscope, 10x objective) and representative images were taken. Bar, 5 μM.
Panel C: Caspase activation after 7Me-IEITC treatment. SKOV-3 cells were treated with 4μM 7Me-IEITC for 1hr, 6hrs, or 18hrs. Analysis of the expression of proteins in the lysates of treated and untreated cells was carried out by PAGE and Western Blot analysis as described (Material and Methods). Primary antibodies against activated caspase-3, -8, -9, and inactivated/cleaved PARP-1 were used. As an internal standard for equal loading the blots were probed with an anti-β-Actin antibody. As a size standard pre-stained Kaleidoscope (Biorad, Hercules, CA) marker was used.
Panel D: Effect of caspase inhibitors on SKOV-3 viability after 7Me-IEITC treatment. SKOV-3 cells were pre-incubated with specific inhibitors (40μM) against pan-caspase, capase-3, caspase-8, or caspase-9 for 2hrs and treated with 7Me-IEITC (0, 2.5 or 5μM) in the continued presence of the inhibitors (40μM) for additional 48hrs. The MTS viability assay was carried out as described (Materials and Methods). Experiments were performed in triplicates; data are expressed as the mean of the triplicate determinations (X±SD) of a representative experiment in % cell viability of samples with untreated cells.
Panel E: TUNEL assay. SKOV-3 cells were treated with either 4μM 7Me-IEITC or 25μM Actinomycin D for 48hrs. Labeling of DNA nicks with fluorescein-12-dUTP and chromatin counterstaining with propidium iodide was carried out as described (Materials and Methods) using a TUNEL assay (Promega, Madison, WI) according to the manufacturer’s recommendations. During fluorescent microscopy (Nikon Eclipse TE2000-E inverted microscope, 10x objective), representative images were taken, apoptotic stain (FL-dUTP, green) and nuclear stain (Pi, red) overlaid; TUNEL positive nuclei due to DNA fragmentation appear as yellow areas. Bar 10 μM.