Table 2.
Tandem retro-DA/DA reactions using bicyclooctenone (−)-3a
| entry | Dienophile(eqiv) | cycloadduct | Time(h) | Yieldb (%) |
|---|---|---|---|---|
| 1 |
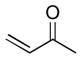 (5 equiv) (5 equiv)9 |
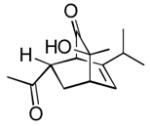 12c 12c
|
3 | 92 |
| 2 |
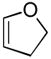 (20 equiv) (20 equiv)10 |
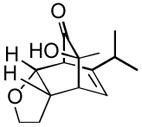 13 13
|
12 | 84 |
| 3 |
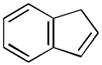 (10 equiv) (10 equiv)11 |
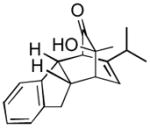 14 14
|
3 | 99 |
| 4d |
15 |
 16 16
|
4 | 42 |
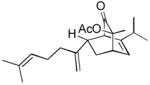
 17 17
|
4 | 39 | ||
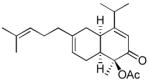
| 5 |
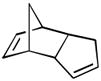 (5 equiv) (5 equiv)18 |
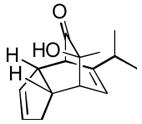 19 19
|
4 | 98 |
| 6 |
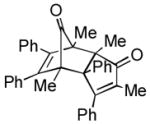 (2.5 equiv) (2.5 equiv)20 |
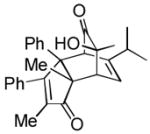 21 21
|
5 | 57 |
Reaction conditions: dimer (−)-3, dienophile, mesitylene, 150 °C;
Isolated yield after column chromatography;
Approximately 6% of an inseparable minor product was observed by 1H-NMR;
Acetylation required for product separation.
