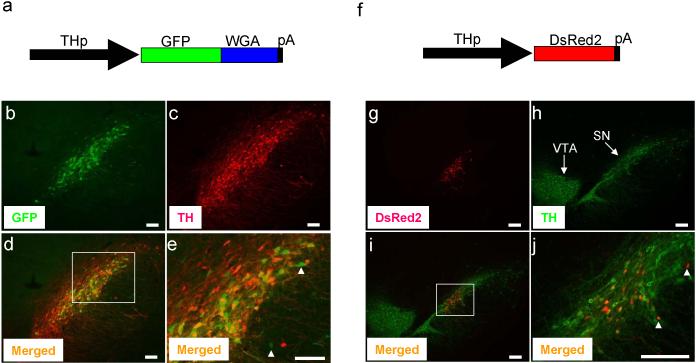
Figure 3.
Transgenes were expressed in SN of adult rat. AAV (1.5 μl of 2-5 × 1012 genome copy/ml) which expresses GFP-WGA fusion protein (a) or DsRed2 (f) was delivered to the SN by stereotaxic injection as described in Figure 2. Seven or 17 days after injection, animals were analyzed. Expression of GFP-WGA (b) and DsRed2 (g) was detected by its fluorescence. TH expression was detected by immunostaining (c, h). Merged images of GFPWGA and TH (d) or DsRed2 and TH (i) are shown. Magnified view of boxed area of (d) and (i) are shown in (e) and (j), respectively. Expression transgenes in the non-DA cells are shown by white arrow heads. VTA and SN are shown. Bar; 100 μm (b, c, d, e), 200 μm (g, h, i, j). Goat anti-WGA antibody (1:200 dilution, Vector Lab) and rabbit anti-TH antibody (1:1,000 dilution, Pel-Freez) were used. For immunofluorescence labeling, Alexa 594 or 488 anti-rabbit or anti-goat IgG (1:200 dilution, Molecular Probes) were used as secondary antibodies. Recombinant AAV was obtained by using the AAV helper-free system according to manufacture's protocol (Stratagene). We replaced the CMV promoter of pAAV-MCS with the 2.5 kb rat TH promoter to obtain the pAAV2.5THp. The GFP-WGA fusion protein or DsRed2 were cloned in the pAAV2.5THp. This plasmid was used to transfect AAV-293 cells with pAAV-RC and pHelper using the calcium phosphate method to make recombinant viral particles. Viruses were harvested 3 days after transfection and purified using heparin-agarose column 21 and concentrated by centrifugation using a filter membrane (Amicon). One ml of viral solution was treated with proteinase K and viral DNA was recovered by phenol extraction followed by ethanol precipitation. The genome copy of viral DNA was determined by PCR.