Fig. 2.
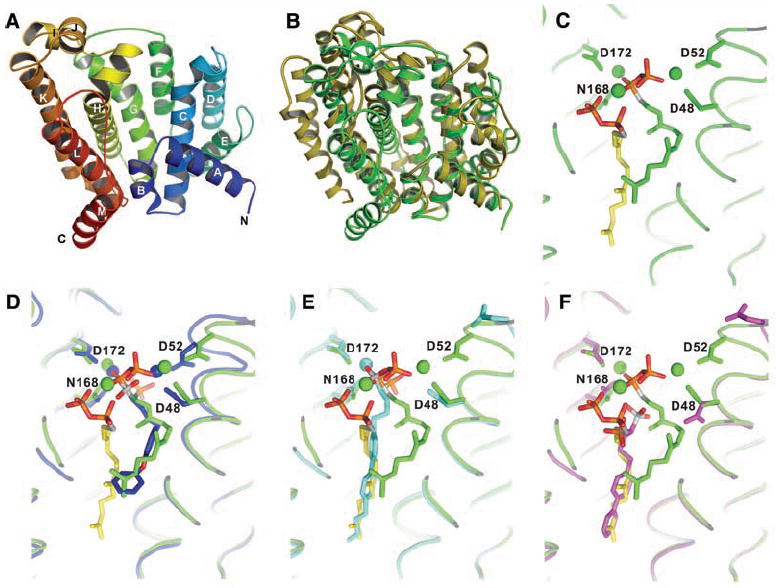
X-ray crystallographic structures. (A) X-ray structure of S. aureus CrtM. (B) Superposition of CrtM and human squalene synthase structures. There is a 5.5 Å Cα RMS deviation between the two structures. (C) Close-up view of FsPP bound to CrtM. (D) Close-up view of S. aureus CrtM with bound BPH-652. (E) S. aureus CrtM with bound BPH-698. (F) S. aureus CrtM with bound BPH-700. In (C) to (F), the FsPP ligands are in green or yellow; BPH-652, BPH-698, and BPH-700 (and associated Mg2+) are in blue, cyan, and magenta, respectively. Key contacts with Asp (D) and Asn (N) residues are indicated.
