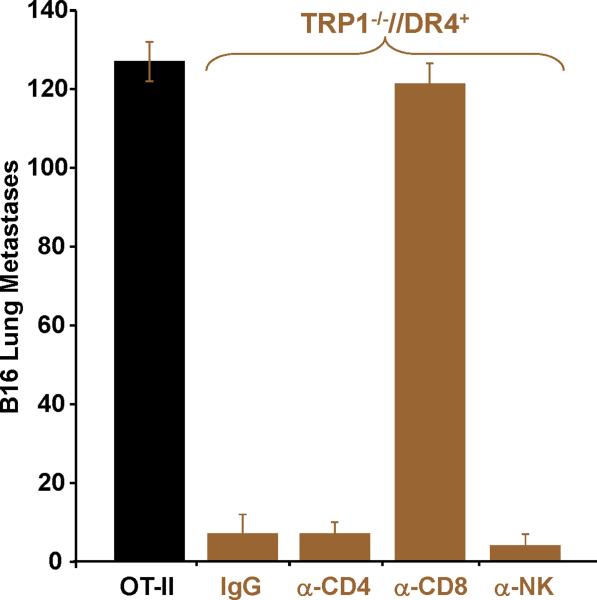
Figure 4.
Adoptively transferred CD4+ T cells derived from TRP-1 KO mice eradicate established tumor by priming host CD8+ T cells. To prepare cells for adoptive transfer, TRP1-/-//DR4+ and TRP1+/-//DR4+ Tg littermates were immunized with hTRP-1 protein and expanded in vitro. Control OT-II mice were similarly immunized and expanded. (A) A general schematic of all tumor treatment experiments. (B) Treatment is antigen-specific and DR4-restricted. Expanded T cells were adoptively transferred (107 per mouse) into three different tumor-bearing hosts at 5 mice per group: 1) B16 (TRP-1+) lung metastases in DR4 transgenics (DR4+); 2) B16 lung metastases in C57BL/6 mice (DR4-); 3) MC38 (TRP-1-) lung metastases in DR4 transgenics. Each group had been previously IV tail vein inoculated with tumor. CD4+ T cells derived from the TRP-1-/-//DR4+ mice nearly fully eradicate the 4-day lung metastases (mean = 5; p = 0.0006 using a two-tailed Student T-test) when compared to littermate (WT) controls (mean = 68) and control OVA-specific CD4+ T cells (mean: 156). No treatment effect was observed (mean = >150 lung metastases for all treatment groups) in mice bearing the control tumor cell line (MC38) or in recipient mice lacking the specific restriction element (C57BL/6 mice; DR4-). (C) Treatment of B16 lung metastases is dependent upon host CD8+ T cells. Tumor bearing (B16 only) DR4 Tg mice were depleted of individual effector-cell subsets (CD4, CD8, NK and NKT) following IP injection of mAb's prior to adoptive transfer of CD4+ T cells derived from TRP1-/-//DR4+ mice. Control OVA-specific CD4+ T cells were used to measure the non-specific effects of adoptive transfer. Each experimental arm involved 5 mice per group and all experiments were performed 2-3 times with similar results.