FIG. 1.
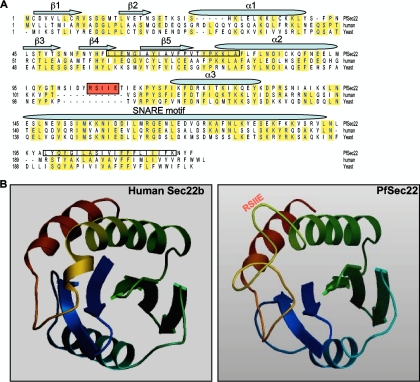
Sequence features of PfSec22. (A) Sequence alignment of PfSec22 with its homologues in humans and yeast. Identical amino acids are highlighted in yellow, while the PEXEL-like motif is shaded in red. The five beta strands (β1 to β5) and three alpha helix (α1 to α3) structures of the longin domain are indicated. The predicted hydrophobic segments (N-terminal and C-terminal hydrophobic segments) are boxed. PfSec22 and human Sec22b are 30.9% identical; PfSec22 and yeast Sec22p are 26.2% identical. In comparison, the human and yeast Sec22 polypeptides are 37.2% identical. (B) Ribbon structures of human Sec22b and PfSec22 longin domains showing the locations of the PEXEL-like motif (RSIIE). The PfSec22 structure was modeled with SWISS-MODEL 8.05 using the human Sec22b sequence as a template.