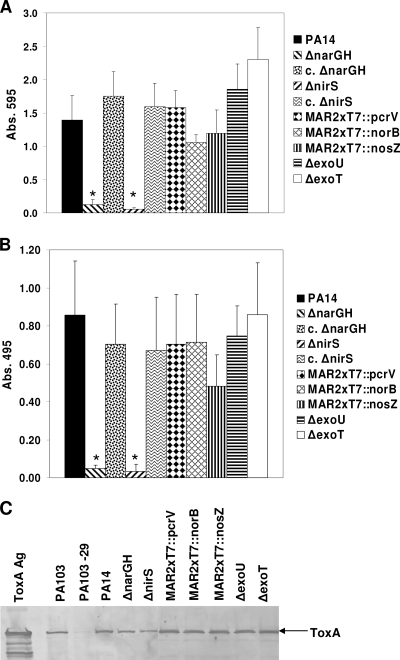
FIG. 6.
Total protease, elastase, and exotoxin A secretion by denitrification pathway mutants. Culture supernatants from wild-type PA14 and denitrification pathway and T3SS mutants were compared for total protease (A) and elastase (B) activities. Only the ΔnarGH and ΔnirS mutants displayed significantly lower levels of protease and elastase secretion than wild-type PA14. Protease secretion was restored to normal in the genetically complemented ΔnarGH and ΔnirS mutant strains (c. ΔnarGH and c. ΔnirS, respectively). Supernatants from each strain were tested in triplicate in three separate experiments, and the data represent the mean ± SD. In contrast, wild-type PA14 and all of the mutant strains derived from PA14 secreted ToxA into their culture supernatants, as detected by Western blotting (C). P. aeruginosa PA103 and PA103-29 were used as positive and negative controls, respectively, for the production of exotoxin A; ToxA Ag is the purified P. aeruginosa exotoxin A (1 μg) used as a positive control for Western blotting. Abs. 495, absorbance at 495 nm.