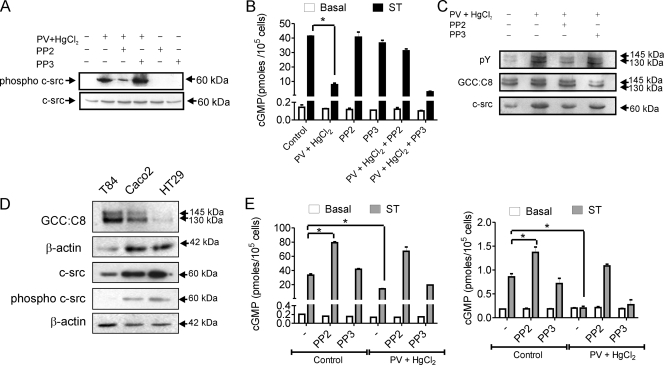
FIG. 2.
Activation of c-src in intestinal cells regulates ligand-mediated cGMP production by GC-C, (A) T84 cells were treated as indicated and lysates prepared from cells subjected to Western blot analysis to monitor levels of active c-src (phospho-c-src antibody) and total c-src. The data shown are representative of experiments repeated thrice. (B) T84 cells were treated with HgCl2 and PV following PP2 or PP3 pretreatment for 30 min. cGMP production on addition of ST (100 nM) was measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean of duplicate determinations in experiments repeated thrice (*, P < 0.01). (C) GC-C was immunoprecipitated from T84 cells following treatments as indicated. Immune complexes were subjected to Western blotting with pY and GCC:C8 monoclonal antibodies. To monitor coimmunoprecipitation of c-src, the blot was stripped and then probed with c-src monoclonal antibody. (D) Western blots with lysates from T84, Caco2, and HT29 cells with GCC:C8 monoclonal antibody. The amount of T84 lysate taken was 4 times lower (as was seen with the β-actin loading control) than that of Caco2 and HT29 because of the high expression of GC-C in T84 cells. For Western blotting performed with phospho-c-src and total c-src, whole-cell lysate protein (50 μg) from the three cell lines was used, with β-actin indicating equal loading of the protein. (E) Caco2 (left panel) or HT29 (right panel) cells were treated as indicated and cGMP production on addition of ST (100 nM) measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean of duplicate determinations in experiments repeated thrice (*, P < 0.05).