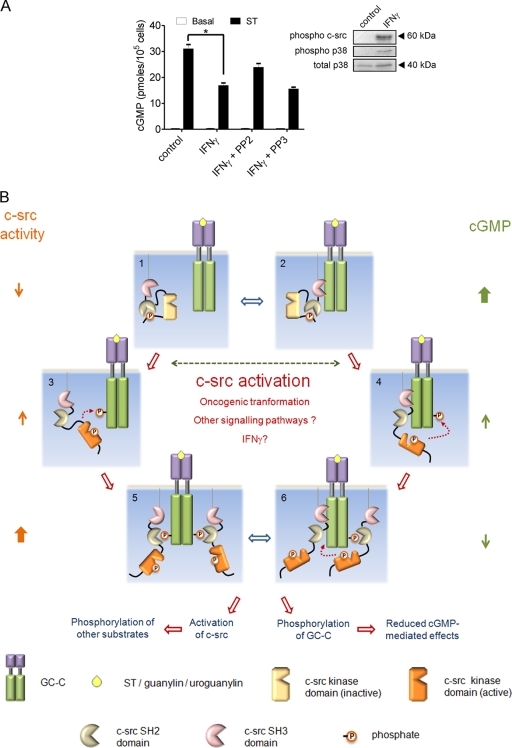
FIG. 6.
GC-C and c-src cross talk in the intestinal cell. (A) T84 cells were treated with IFN-γ (100 ng/ml) for 24 h. Cells were then either lysed for Western blot analysis with the indicated antibodies or treated with ST for 15 min, following which cGMP was measured by radioimmunoassay. Values shown represent the mean ± standard error of the mean for duplicate determinations for assays repeated thrice (*, P < 0.05). (B) Model for GC-C and c-src cross talk. In a normal intestinal cell, c-src is inactive (1) and/or associated with GC-C via its SH3 domain (2). Under these conditions, most GC-C molecules are unphosphorylated, and on binding ligand (either ST/guanylin/uroguanylin) they produce large amounts of cGMP. Activating mutants of c-src, dysregulated cellular signaling pathways, or ligands (e.g., IFN-γ) contribute to enhanced activation of c-src in the colonic cell. GC-C is now tyrosine phosphorylated at Tyr820 by either free c-src (3) or associated c-src (4). This phosphorylation reduces cGMP production by GC-C. Phosphorylated GC-C can now recruit more c-src via the SH2 domain and, in turn, further phosphorylate GC-C (5 and 6). Binding of c-src to GC-C might also prevent subsequent dephosphorylation of GC-C by cellular phosphatases (5). With an increase in the number of GC-C molecules that are tyrosine phosphorylated, ligand-mediated cGMP production reduces drastically, effectively abrogating the antiproliferative actions of the ligands of GC-C. The c-src activated by interaction with GC-C can now phosphorylate a number of cellular substrates and modulate cell division, migration, and invasiveness.