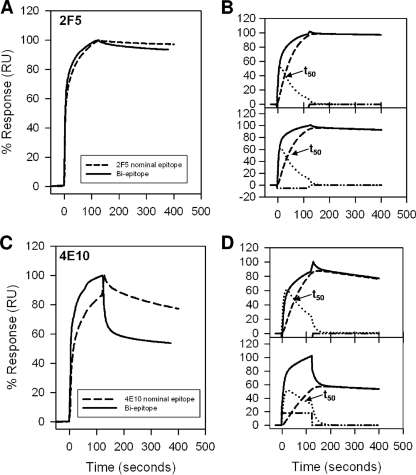
FIG. 2.
Interaction of MAbs 2F5 and 4E10 with MPER peptide-liposome conjugates. (A) Results for binding of MAb 2F5 to 2F5 nominal epitope peptide-liposome conjugate and biepitope peptide-liposome conjugate are shown. RU, resonance unit. (B) The binding of MAb 2F5 to 2F5 nominal epitope (top) and biepitope (bottom) peptide-liposome conjugates follows the two-step conformational change model. (C and D) Binding of MAb 4E10 to the 4E10 nominal epitope and biepitope peptide-liposome conjugates as described for panels A and B. In each of the overlays (B and D), the binding data are shown by solid lines and represent the observed total binding response. The component curves for the encounter (dotted lines) and docked complexes (dashed lines) were simulated from the experimentally determined rate constants (Table 1). t50 is the time required for half of the encounter complex to be converted to docked complex.