Abstract
Venezuelan equine encephalitis virus (VEEV) is highly virulent in adult laboratory mice, while Sindbis virus (SINV) is avirulent regardless of dose or inoculation route, dependent upon functioning alpha/beta interferon (IFN-α/β) responses. We have examined each virus' resistance to and/or antagonism of IFN-α/β responses in neurons, a cell type targeted by both viruses in mice, by infecting IFN-α/β-treated or untreated primary cultures with viruses or virus-derived replicons that lacked the structural proteins. Priming with IFN-α/β prior to infection revealed that VEEV replication and progeny virion production were resistant to an established antiviral state while those of SINV were more sensitive. Postinfection IFN-α/β treatment revealed that phosphorylation of STAT1 and STAT2 was partially blocked by infection with either virus, dependent upon expression of nonstructural proteins (nsP), but not structural proteins (sP). However, inhibition of STAT phosphorylation by VEEV replicons was not correlated with inhibition of IFN-stimulated gene (ISG) mRNA induction, yet ISG induction was inhibited when sP were present. Host translation was inhibited by VEEV nsP even when cells were pretreated with IFN-α/β. SINV blocked ISG induction and translation, associated with nsP-mediated shutoff of macromolecular synthesis, but both activities were sensitive to IFN-α/β pretreatment. We conclude that both VEEV and SINV limit ISG induction in infected neurons through shutoff of host transcription and translation but that inhibition by VEEV is more resistant to IFN-α/β priming. Likewise, both viruses inhibit IFN receptor-initiated signaling, although the effect upon host responses is not clear. Finally, VEEV appears to be more resistant to effectors of the preestablished antiviral state.
Venezuelan equine encephalitis virus (VEEV) and Sindbis virus (SINV) are members of the Alphavirus genus in the Togaviridae family of mosquito-borne, positive-sense RNA viruses. Members of this genus are responsible for millions of human infections annually and, occasionally, epidemic outbreaks, such as the current widespread infections with Chikungunya virus (CHIKV) in the Indian Ocean territories. The so-called “Old World” viruses, which include prototypic SINV as well as CHIKV, O'nyong nyong virus, and Semliki Forest virus, generally cause a mild to moderate febrile illness in humans that, depending upon the virus, may lead to persistent arthralgia. However, in the recent CHIKV outbreak, evidence of hemorrhagic fever and encephalitis was observed in severely ill humans (5, 8, 32, 39). The “New World” viruses, which include VEEV, Eastern equine encephalitis virus (EEEV) and Western equine encephalitis virus, can also cause a febrile illness; however, infection progresses to encephalitis in a high percentage of cases (25).
For many years, infection of mice and cultured cells has been used to model alphavirus interactions with particular cell types and the effects of virus infection upon host innate immune and antiviral responses (25). Studies by several groups (1, 17, 19-21) have suggested that an important component of the alphavirus interaction with murine and other cells is the virus-mediated arrest of host transcription and translation, which greatly limits the capability of the cell to upregulate expression of alpha/beta interferon (IFN-α/β) genes in response to virus infection. Furthermore, although not yet demonstrated, it is also possible that this mechanism limits IFN-α/β-mediated antiviral effector gene upregulation in infected cells that is mediated through type I IFN receptor signaling. Old and New World viruses appear to achieve these effects through the actions of different proteins, with nonstructural protein 2 (nsP2) of Old World viruses implicated in both transcription and translation shutoff (17, 20, 23) and the capsid structural protein (sP) of New World viruses implicated in transcription shutoff (1, 19, 21). Mechanisms of translation arrest in New World virus infections have not been investigated. However, although these in vitro studies suggest similarities between the alphaviruses in the capacity for host shutoff, this does not translate directly to virulence in mice, as wild-type strains of encephalitic New World viruses cause fatal disease in adult mice but wild-type Old World viruses generally do not (25). Yet, when IFN-α/β or IFN-α/β and IFN-γ signaling is disrupted in mice through deletion of receptor subunits, Old World viruses such as SINV, SFV, and CHIKV cause fatal disease in adult animals (11, 16, 45, 46, 48). This suggests that relative resistance to the IFN-mediated antiviral response underlies important differences in alphavirus disease-causing potential, but the relationship of host macromolecular-synthesis shutoff to IFN resistance in vivo is not clear. Previous work from our laboratory (44) and by others (38) has demonstrated that VEEV replication is more resistant than SINV to the effects of IFN-α/β priming in simple models in which fibroblast cells are pretreated with IFN-α/β, yielding protection from a cytopathic effect. However, the effects of VEEV or SINV infection upon the totality of the IFN-α/β-induced antiviral response in cells relevant to virus disease in vivo have not been examined, including IFN-α/β production by infected cells, effects of infection upon IFN-α/β receptor signaling and subsequent antiviral gene upregulation, or the characteristics of resistance/sensitivity of the viruses to the preexisting antiviral state.
In the current studies, we compared the interactions of VEEV and SINV with the inductive and effector phases of the IFN-α/β antiviral response in primary mouse cortical neuron cultures. Consistent with previously reported results using cultured fibroblasts (17, 21; C. W. Burke, C. L. Gardner, K. D. Ryman, and W. B. Klimstra, submitted for publication), SINV and VEEV suppressed both the production of IFN-α/β and the upregulation of antiviral effector IFN-stimulated genes (ISGs) in neurons, correlated with shutoff of host transcription and/or translation early after infection. We also observed that VEEV gene expression was more resistant than SINV to the antiviral actions of a preexisting IFN-induced antiviral state and VEEV could replicate efficiently under conditions where SINV replication was greatly reduced. Finally, infection with both viruses partially blocked phosphorylation of STAT1 and STAT2, transcription factors involved in the JAK-STAT signaling pathway activated by IFN-α/β receptor signaling (reviewed in references 6, 51, and 52). Together, these data suggest that while both SINV and VEEV can rapidly suppress innate responses in unprimed murine neurons through shutoff of host cell macromolecular synthesis and can partially block IFN-α/β receptor signaling cascades, the enhanced virulence of VEEV in the infected animal may result from effective suppression of host responses even in the face of exposure of cells to IFN-α/β prior to infection, combined with greater resistance to or avoidance of effectors of the antiviral state.
MATERIALS AND METHODS
Cell culture.
BHK cells were maintained in AlphaMEM (Mediatech) supplemented with 10% donor calf serum, 200 mM l-glutamine (Sigma), 10,000 units/ml penicillin G sodium (Sigma), 10 mg/ml streptomycin sulfate (Sigma). Vero cells were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% fetal bovine serum (FBS) and supplements as with BHK cells. All cells were grown at 37°C in a humidified chamber with 5% CO2.
For neuron cultures, pregnant (gestation day 13 to 15) CD1 mice were purchased from Charles River Laboratories. Mice were housed in the Animal Resources Center (Louisiana State University Health Sciences Center, Shreveport) under specific-pathogen-free conditions. All procedures were carried out in accordance with federal and institutional guidelines for animal care and use.
Primary mouse cortical neuron cultures were prepared from CD-1 mice at gestation days 13 to 16 as described in references 36 and 49, with modifications. Briefly, brains were removed from the embryos into calcium/magnesium-free Hanks balanced salt solution (Sigma) with glucose and gentamicin (20 μg/ml) and subsequently incubated at 37°C for 20 min in enzyme digestion solution (Hanks balanced salt solution containing 1 mg/ml papain, 50 μg/ml DNase I, 0.2 mg/ml l-cysteine [Sigma], 1.5 mM CaCl2, and 0.5 mM EDTA [Invitrogen]) with agitation every 5 min. After supernatant removal, the papain was neutralized by addition of excess DMEM (Invitrogen) supplemented with 10% FBS. Digested tissue was then centrifuged and medium removed followed by addition of 5 ml neuron culture medium (neural basal medium supplemented with 2% B27, 0.5 mM glutamine, and 20 μg/ml gentamicin) (Invitrogen), and tissues were pipetted 20 times to disaggregate cells. Twenty-five ml of neuron culture medium was added and cells were then pelleted (1,000 rpm, 5 min) and resuspended in 10 ml neuron culture medium and enumerated by trypan blue exclusion. Plates for neuron cultures were coated with a 30-μg/ml solution of poly-d-lysine (Sigma) for either 2 to 6 h at room temperature or at 4°C overnight followed by phosphate-buffered saline rinse and drying. Coating was completed immediately before neuron culture. Tissue culture plates were seeded at either 2.5 × 105 cells per well (24-well plates) or 1.5 × 106 cells per well (6-well plates). Neuron culture medium was replaced at 24 h after initial culture and at 2-day intervals thereafter. Cells were used for experiments after 5 to 6 days in culture. The cultures were stained with the anti-NeuN antibody (Chemicon Millipore) and found to be >95% pure.
Viruses and replicons.
Construction of the VEEV ZPC738 cDNA clone (2), the V3000 Trinidad donkey cDNA clone (15), the V3000 tripartite replicon system (41), and the SINV TR339 cDNA clone and tripartite replicon system (30) have been described previously. The TR339 replicons were modified to express murine IFN-α4 or IFN-β genes by amplification of the genes from SINV-infected mouse dendritic cell total RNA using specific primers that introduced XbaI and NotI restriction sites on the 5′ and 3′ ends, respectively. Appropriately restriction enzyme-digested fragments were then subcloned into the TR339 replicon vector. Propagation-competent parental viruses were produced by electroporation of in vitro-synthesized RNA into BHK cells as described previously (2, 15, 30). Supernatants were harvested at 24 h postelectroporation, clarified by centrifugation, and stored at −80°C. Viruses titers were determined by conventional plaque assay on BHK cells. Replicons were produced as described previously (18, 30, 42) by coelectroporation of 20 μg of each of the three component RNAs (replicon genome, capsid helper, and glycoprotien helper) followed by harvesting and processing of supernatants as described for parental viruses. Green fluorescent protein (GFP)-expressing replicon stock titers were determined on BHK cells by using fluorescence microscopy.
Neuron infection, interferon treatment, and interferon assay.
Neurons were infected with viruses or replicons at the multiplicities indicated in the figure legends. For STAT phosphorylation inhibition and reverse transcription-PCR (RT-PCR) assays, infections of neurons were normalized using GFP-expressing versions of each replicon and fluorescence microscopy analysis such that >95% of the neurons were infected and similar infectious doses of each virus/replicon were delivered in all cases. Relative neuron infectious dose was calculated for each virus by determining the minimal number of BHK infectious units required to infect 95% of the neurons and then utilizing that dose for all infections. Virus production in growth assays was determined by calculating titers via plaque assay with BHK cells. The significance of differences was determined by using Student's t test for unpaired samples.
IFN-α/β stocks were derived from electroporation of BHK cells with SINV replicon vectors expressing the murine IFN-β or IFN-α4 genes. At 12 to 18 h postelectroporation, supernatants were harvested and clarified initially by centrifugation at 5,000 rpm for 40 min followed by ultracentrifugation for 18 h at 24,000 rpm in an AH-629 rotor. After centrifugation, the pH of stocks was lowered to 2.0 by using hydrochloric acid and stocks were stored at 4°C. After 24 h, the pH was raised to 7.0 using sodium hydroxide and equal volumes of the IFN-α and IFN-β stocks were mixed. Subsequently, IFN-α/β IU titers were determined by biological assay using L929 cells, encephalomyocarditis virus, and an IFN-α/β standard (Access Biomolecular) as previously described (55). Specificity of the IFN preparation was assessed by performing RT-PCR for ISG induction after exposure of cells derived from type 1 IFN receptor-deficient IFNAR1−/− mice to high concentrations of the preparations. No induction of ISG mRNA or antiviral activity versus SINV was observed.
Western blotting.
Total cell extracts were made from primary neurons and used for Western blot analysis. Cells were lysed in radioimmunoprecipitation assay buffer (50 mM Tris, pH 7.4, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate [SDS], 1 mM EDTA, 1 mM EGTA) supplemented with protease inhibitors (1 mM phenylmethylsulfonyl fluoride, 1 μg/ml leupeptin, and 1 μg/ml pepstatin), and phosphatase inhibitor cocktail (Sigma). Protein concentrations were determined using a bicinchoninic acid assay (Pierce). Approximately 20 μg of protein lysate was resolved per lane of an SDS-polyacrylamide gel electrophoresis (SDS-PAGE) gel (ranging from 7% to 12%) and transferred to a nitrocellulose membrane (Bio-Rad). Membranes were blocked for at least 1 h in 5% skim milk in Tris-buffered saline (TBS)-0.1% Tween 20 (TBST) followed by overnight incubation with primary antibodies at 4°C. Antibodies were diluted in TBST with 3% bovine serum albumin (BSA) according to the manufacturer's guidelines. Following four sequential 15-min washes in TBST, membranes were incubated with horseradish peroxidase-conjugated secondary antibody (Zymed) diluted in TBST with 2% skim milk. Bound secondary antibody was detected using the ECL-Plus chemiluminescence system (Amersham).
The membranes were probed with the following antibodies: rabbit polyclonal antibodies specific for p-Tyr701 STAT1 (Cell Signaling), STAT1 (M-22; Santa Cruz Biotechnology), p-Tyr689 STAT2 (Upstate Biotechnology/Millipore), STAT2 (H-190; Santa Cruz), murine monoclonal antibodies specific for β-actin (Chemicon) and GFP (Invitrogen), and murine hyperimmune ascitic fluid for the capsid of SINV and VEEV (ATCC). All Western blot assays were performed at least three times with similar results.
Semiquantitative RT-PCR.
For semiquantitative RT-PCR, total cellular RNA was isolated using the TRI reagent (Molecular Research Center, Inc.). Approximately 1.5 μg of total RNA per sample was reverse transcribed using random hexamer primers. Equal volumes of each cDNA were then subjected to PCR amplification with murine gene-specific primers (Integrated DNA Technologies) designed against GenBank sequences of each gene (ISG20, ISG15, ISG56, ZAP, viperin, and β-actin) as previously described (57). Either β-actin or 18S rRNA PCR was used as a loading control (18S primer sequences: sense, 5′-CGCCGCTAGAGGTGAATTTCT-3′; antisense, 5′-CGAACCTCCGACTTTCGTTCT-3′). The volume of the cDNA template included in these reaction mixtures and the number of amplification cycles were optimized to ensure that reactions were stopped during the linear phase of product amplification, permitting semiquantitative comparisons of mRNA abundance between different RNA preparations. To exclude the possibility of contaminating DNA, control reactions were performed in parallel in the absence of reverse transcriptase. RT-PCR products were resolved by agarose gel electrophoresis and visualized on a VersaDoc 4000 imaging system (Bio-Rad). Reproducibility of results was confirmed by performance of RT-PCR analyses at least three times using RNA derived from different neuron preparations. Semiquantitative RT-PCR was used for these studies because other techniques were not available to us in the biosafety level 3 containment laboratory.
Metabolic labeling of neurons.
Neuron cultures were either untreated or treated with IFN and then either left uninfected or infected with SINV and VEEV or replicons as described above and in the figure legends. Ten minutes prior to labeling, neuron media were removed and replaced with starvation medium (Met/Cys-free DMEM [Invitrogen] supplemented with 1% FBS and glutamine and penicillin-streptomycin as in the neuron culture medium). Then, 100 μCi/ml of [35S]Met-Cys (Amersham) was added for 2 h and incubation continued at 37°C. Cells were then washed once with phosphate-buffered saline and lysed using radioimmunoprecipitation assay buffer. Equal volumes of radiolabeled lysates were then run on 10% SDS-PAGE gels, and fixed and dried gels were exposed to film for visualization of radiolabeled proteins. Similarity in the original cell number in the lysate volumes loaded was confirmed by Western blotting for β-actin, the abundance of which does not vary substantially during virus-mediated host macromolecular synthesis shutoff.
RESULTS
Effects upon virus replication of IFN-α/β pre- or postinfection treatment of neurons.
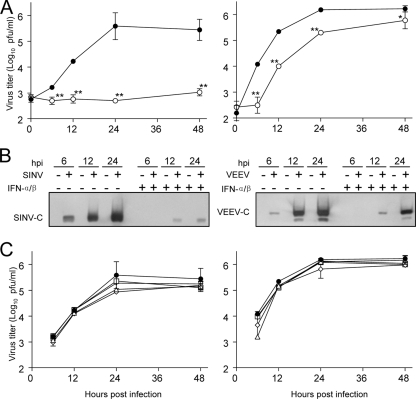
Initially, we wished to determine the effects of IFN-α/β preinfection or postinfection treatment of neurons upon the replication of SINV and VEEV. When neuron cultures were treated with 1,000 IU of IFN-α/β for 24 h prior to high-multiplicity infection, the replication of SINV, as measured by PFU production, was inhibited by ∼150-fold; however, VEEV replication was inhibited only ∼10-fold after an initial lag in replication, measured at 6 h postinfection (p.i.) (Fig. 1A). An initial IFN-α/β-mediated lag in replication was not unexpected for VEEV, as we have previously demonstrated that the virus is sensitive to PKR-independent, IFN-α/β-primed activities that act to inhibit translation initiation of the infecting genome (54). The effective blockade of SINV replication (and the limited blockade of VEEV) could be detected at the point of sP expression, as capsid protein levels increased between 12 and 24 h p.i. for VEEV but little to no increase was detected for SINV (Fig. 1B). In contrast, when cells were treated with the same dose of IFN-α/β either simultaneously or 3, 6, or 12 h after infection, a much-diminished antiviral effect against either virus was observed; although in some instances, statistically significant (P < 0.05) decreases in PFU production versus untreated cells were detected with both viruses (Fig. 1C). These results indicate that production of SINV sP and progeny virus release are substantially more sensitive to the preestablished antiviral state in neurons than those of VEEV; however, both viruses appear to be largely resistant to the effects of IFN-α/β treatment once infection is established.
FIG. 1.
Sensitivity of VEEV or SINV replication to IFN-α/β treatment prior to or after infection of neurons. (A) Neuron cultures that were either untreated (solid circles) or treated with 1,000 IU/ml of IFN-α/β for 24 h (open circles) and then were infected with a BHK 21 cell multiplicity of infection of 3 by each virus, and virus replication was quantitated by plaque assay titration at the times indicated. Each datum point is an average of three infections. *, P < 0.05; **, P < 0.01. (B) Neuron cultures were mock infected or infected with viruses as described in Materials and Methods and harvested for Western blotting to detect SINV or VEEV capsid proteins at the times indicated. Equal protein was loaded in all lanes. (C) Untreated neuron cultures were infected with SINV (left panel) or VEEV (right panel) as in panel A and either left untreated (closed circles) or exposed to 1,000 IU/ml of IFN-α/β at 0 (open triangles), 3 (open diamonds), 6 (open boxes), or 12 h p.i. (open inverted triangles). Supernatants titers were determined at the times postinfection listed on the x axis. All error bars in this figure are standard deviations.
The majority of the IFN-α/β-upregulated antialphavirus activity in neurons is STAT1 dependent.
The results of the previous experiments suggest that both viruses interfere with the establishment of the IFN-α/β-mediated antiviral state after infection of neurons is established. One mechanism for interference with establishment of an antiviral state in infected cells is through blockade of the IFN-α/β receptor-stimulated phosphorylation cascade. A number of viruses have been shown to block the IFN-α/β receptor-mediated activation of the JAK or Tyk initiating kinases or their targets, STAT1 and STAT2 transcription factors, which, once activated by phosphorylation, translocate to the nucleus and participate in transcriptional upregulation of ISGs (reviewed in references 6, 51, and 52).
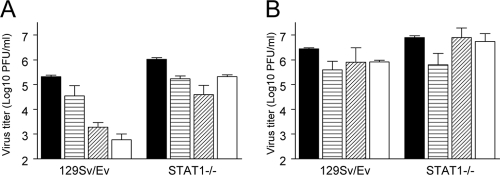
We first established the STAT1 dependence of the antiviral effects in neurons by pretreating with IFN-α/β neurons from normal and STAT1−/− mice and evaluating virion production at 24 h p.i., which was used as a time point representative of the differences in sensitivity of SINV versus VEEV in the original IFN-α/β pretreatment experiment (Fig. 1). IFN-α/β pretreatment of neurons derived from control 129Sv/Ev mice exhibited similar reductions in titer (<10-fold for VEEV and >100-fold for SINV) to cultures derived from CD-1 mice (Fig. 1A and 2A and B). Similar treatment of cultures derived from STAT1−/− mice revealed virtually no anti-VEEV effect and a greatly diminished anti-SINV effect (Fig. 2A and B), although a significant reduction in titer in the IFN-α/β-treated SINV-infected cultures was observed (P < 0.01). Together, these results indicate that in neurons the majority of the antiviral effect versus alphaviruses is STAT1 dependent, although STAT1-independent IFN-α/β-induced activities can partially suppress the replication of SINV.
FIG. 2.
Involvement of STAT1 signaling in the antiviral responses against SINV and VEEV. Neuron cultures from wild-type or STAT1−/− mice were either untreated (solid bars) or treated with 10 (horizontally hatched bars), 100 (diagonally hatched bars), or 1,000 (cross-hatched bars) IU/ml of IFN-α/β for 24 h and infected with a BHK 21 cell multiplicity of infection of 3 with SINV (A) or VEEV (B). Supernatants titers were then determined for virus yield at 24 h p.i. Error bars are standard deviations.
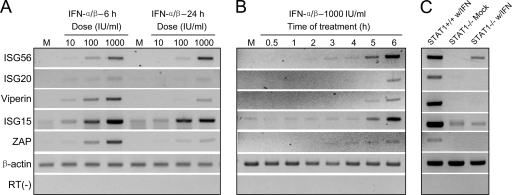
A putative mechanism underlying these results was established by examining the IFN-α/β-induced upregulation of mRNAs for genes we (47, 57) and others (3, 33) previously identified as IFN-α/β upregulated (ISGs) and capable of exerting an antiviral effect against SINV and/or VEEV. We first established the dose response and timing of induction of these ISG mRNAs in normal neurons (Fig. 3A and B), selecting 1,000 IU/ml treatment for all experiments and 6 h after treatment for measurement of ISG induction, since this regimen achieved the most robust induction of all ISGs. In the absence of STAT1, upregulation of only one of the genes examined, ISG56, was observed following IFN-α/β treatment, and the degree of upregulation appeared to be less than observed in normal counterparts (Fig. 3C). We previously demonstrated a moderate anti-SINV activity of p56, the protein derived from the ISG56 mRNA, which may account for at least some of the STAT1-independent anti-SINV activity detected in the current studies and others (22).
FIG. 3.
Timing, dose, and STAT1 dependence of antialphavirus ISG induction. (A and B) Neuron cultures were either untreated (M) or treated with the indicated doses of IFN-α/β for the times indicated. Total cellular RNA was then harvested and subjected to semiquantitative RT-PCR for each ISG and the β-actin control as described in Materials and Methods. (C) Neurons from wild-type (STAT1+/+) or STAT1−/− mice were mock treated or treated with 1,000 IU/ml of IFN-α/β for 6 h, and then RNA was harvested and semiquantitative RT-PCR was performed as described for panels A and B.
Neither SINV nor VEEV infection dismantles the antiviral state in cells exposed to IFN-α/β prior to infection.
To perform experiments examining the phosphorylation states of STAT 1 and STAT2 and the transcriptional activity in the neurons cultures, it was important to establish a multiplicity of infection that resulted in infection of most cells. As described in Materials and Methods, we determined and then subsequently used a multiplicity that achieved >95% infection of the neurons in the first round, based upon examination of neuronal cultures infected with VEEV or SINV GFP-expressing replicons (Fig. 4).
FIG. 4.
Infection of neuron cultures. (A and B) Fluorescence micrographs of neuron cultures infected with a dose of SINV (A) or VEEV (B) GFP-expressing replicons calculated to ensure infection of >95% of cells. (C) Phase-contrast image of untreated neuron cultures. Original magnification in all panels, ×200.
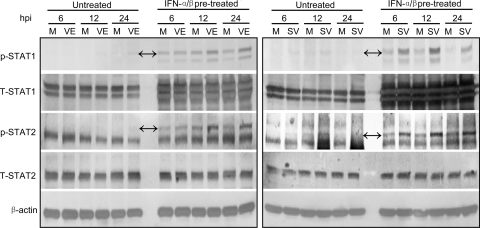
The increased resistance of VEEV to the preexisting antiviral state in neurons could result from a “dismantling” of the antiviral state as has recently been described for paramyxoviruses (40). In this model, it was presumed that sustained antiviral responses required continuous STAT-mediated signaling, which was diminished by viral antagonists via degradation or dephosphorylation of the STAT proteins. To investigate this possibility, we examined the activation cascade that leads to STAT1-dependent gene upregulation after IFN-α/β signaling by assessing the abundance and phosphorylation states of STAT1 (phospho-Tyr701) and STAT2 (phospho-Tyr689) transcription factors that are likely important in the antialphavirus response in neurons (Fig. 2) (7). Neurons were mock treated or IFN-α/β pretreated for 24 h, followed by infection with VEEV or SINV and examination of protein phosphorylation at 6, 12, or 24 h p.i. to determine the effects of infection upon a preexisting antiviral state.
Infection of untreated cells with either of the viruses resulted in limited STAT1 phosphorylation at most times examined (Fig. 5, untreated), suggesting that IFN-α/β production was not robust in response to virus infection and/or that STAT1 phosphorylation was blocked by both viruses. No secreted IFN-α/β could be detected in SINV- or VEEV-infected culture supernatants by biological assay at 6, 12, 18, or 24 h p.i. (limit of detection was 3.9 IU/ml for VEEV and 7.8 IU/ml for SINV), suggesting that the former was true. However, this did not exclude the possibility that blockade of STAT phosphorylation was occurring as well.
FIG. 5.
Effects of VEEV or SINV infection upon STAT1 or STAT2 phosphorylation and abundance in IFN-pretreated neurons. Neuron cultures were either untreated or treated with 1,000 IU/ml of IFN-α/β for 24 h and then either mock infected (M) or infected with VEEV (VE; left panel) or SINV (SV; right panel) as described in Materials and Methods. Lysates were harvested at the times after infection indicated and processed for Western blotting for the indicated proteins (p-STAT1/2, phosphorylated STAT1/2; T-STAT1/2, total STAT1/2) as described in Materials and Methods. In panels with nonspecific staining, double arrows indicate the protein bands of interest.
In uninfected neurons pretreated with IFN-α/β, an increase in STAT1 abundance and phosphorylation over untreated controls was observed at all times, as expected. Infection of pretreated cells with either virus had no reductive effect upon the abundance of STAT1 or its phosphorylation state at any time postinfection (Fig. 5, IFN-α/β pretreated). In fact, infection with both viruses increased the phosphorylation of STAT1 over pretreated, mock-infected cells. A similar pattern was observed with STAT2 (Fig. 5). These results indicate that SINV and VEEV do not decrease the amount of STAT1 in infected neurons and that the viruses actually increase the extent of phosphorylation of these proteins versus uninfected cells if the cell is exposed to IFN-α/β prior to infection. Therefore, it is unlikely that the enhanced resistance of VEEV to the antiviral state in IFN-pretreated cells arises from “dismantling” of the STAT-dependent antiviral state.
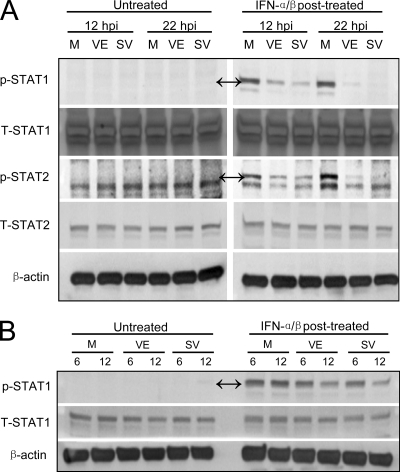
VEEV and SINV block new STAT1 and STAT2 phosphorylation in infected neurons.
In our initial experiments, VEEV and SINV were largely resistant to the antiviral effects of IFN-α/β when it was added after infection had been established (Fig. 1C), perhaps implying an effect upon STAT signaling after viral proteins are produced. To examine this possibility, we infected untreated cultures followed by comparison of STAT abundance and phosphorylation after IFN-α/β treatment for 30 min at either 12 or 22 h p.i. This approach permitted assessment of the effects of virus replication intermediates upon the initiation of the antiviral state.
When cells were treated with IFN-α/β for 30 min at various times after infection with either virus, no effects upon the abundance of STAT1 or STAT2 were detected; although STAT1 is induced by IFN-α/β in the neuronal cultures, it is unlikely that 30 min is sufficient time for protein expression. However, compared to mock-infected, IFN-α/β-treated controls, phosphorylation of both transcription factors was slightly reduced in cells treated at 12 h p.i. and substantially reduced at 22 h p.i. (Fig. 6A). We also examined the timing of inhibition after infection and determined that blockade of STAT1 phosphorylation was first detectable between 6 and 12 h p.i. with both viruses (Fig. 6B). Together with the results of the previous section, we conclude that both viruses appear to suppress IFN-α/β secretion from neurons in response to infection and also to largely block STAT pathway activation if virus replication is initiated before cells are exposed to IFN-α/β, but not in cells that are primed before infection. We attempted to use immunocytochemistry to determine if nuclear translocation of STAT1 and STAT2 was also blocked by virus infection, but the proteins could not be reliably detected by this technique in the primary neuron cultures.
FIG. 6.
Effects of VEEV or SINV infection upon STAT1 or STAT2 phosphorylation and abundance in IFN-posttreated neurons. Neuron cultures were either mock infected (M) or infected with VEEV or SINV as described in Materials and Methods and then either left untreated or treated with 1,000 IU/ml of IFN-α/β for 30 min at 12 or 22 h p.i. (A) and 6 or 12 h p.i. (B). Lysates were harvested immediately after IFN-α/β treatment and processed for Western blotting for the indicated proteins as described in Materials and Methods. In panels with nonspecific staining, double arrows indicate the protein bands of interest.
Patterns of ISG upregulation after VEEV or SINV infection.
We next determined whether or not the blockade of STAT1/2 phosphorylation events after virus infection translated into a reduction in the synthesis of IFN-α/β-inducible, antiviral gene mRNAs by performing semiquantitative RT-PCR analyses. Regulation of the IFN-β mRNA was also measured to evaluate the effects of infection upon the gene expression during the inductive phase of the IFN-α/β response. Experiments were set up as above with neurons either untreated or IFN-α/β treated before virus or mock infection, or cells were mock or virus infected and then left untreated or treated with IFN-α/β.
In cells that were not treated with IFN-α/β, SINV infection resulted in very little upregulation of the IFN-β or ISG mRNAs versus mock-infected cells at any time measured (Fig. 7 and 8, untreated). In contrast, VEEV infection modestly upregulated the IFN-β mRNA and multiple ISGs. However, as mentioned above, release of IFN-α/β was not detected by a biological assay after infection with either virus. In separate studies, we have found that infection with SINV or VEEV does not block the cell signaling pathways that lead to IFN-α/β induction in murine fibroblasts prior to the point of transcriptional upregulation (Burke et al., submitted). Considered together, these findings imply that SINV infection inhibits transcription more efficiently than VEEV but that production of the proteins may be impaired after transcription in VEEV-infected and possibly also SINV-infected neurons.
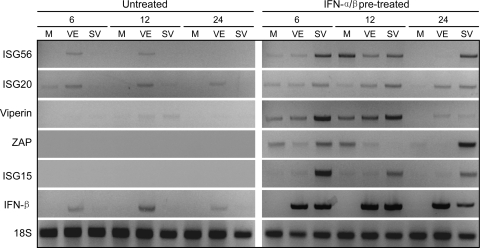
FIG. 7.
Effects of VEEV or SINV infection upon IFN-β gene and ISG transcription in IFN-pretreated neurons. Neuron cultures were either untreated or treated with 1,000 IU/ml of IFN-α/β for 24 h and then either mock infected (M) or infected with VEEV (left panel) or SINV (right panel) as described in Materials and Methods. Total cellular RNA was harvested at the times indicated and processed for semiquantitative RT-PCR for the indicated mRNAs as described in Materials and Methods.
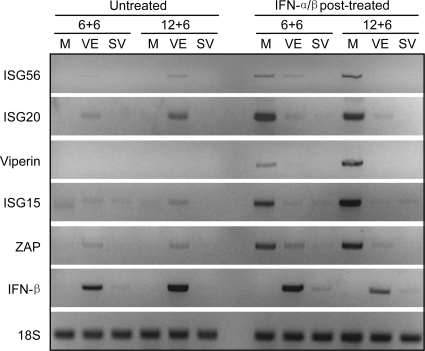
FIG. 8.
Effects of VEEV or SINV infection upon IFN-β gene and ISG transcription in IFN-posttreated neurons. Neuron cultures were either mock infected (M) or infected with VEEV or SINV as described in Materials and Methods and then either left untreated or treated with 1,000 IU/ml of IFN-α/β for 6 h beginning at 6 (6 + 6) or 12 (12 + 6) h p.i. Treatment and harvest times were set to allow gene upregulation and minimize cytopathic effects of viruses upon untreated cells. Total cellular RNA was harvested 6 h after the onset of IFN treatment and processed for semiquantitative RT-PCR for the indicated mRNAs as described in Materials and Methods.
When neurons were pretreated with IFN-α/β for 24 h prior to infection, multiple ISG mRNAs, but not the IFN-β mRNA, were upregulated at early times in mock-infected cells (Fig. 7, compare mock lanes for untreated and IFN-α/β pretreated). SINV infection of pretreated neurons upregulated the IFN-β mRNA and further upregulated multiple ISG mRNAs, although the patterns of upregulated ISGs were not identical at all times examined. In contrast, ISG transcription after VEEV infection of pretreated neurons was generally equivalent to or lower than in pretreated, mock-infected cells, with the exception of the IFN-β mRNA, which was induced to a similar extent as with SINV infection. From these results we infer that, when neurons are exposed to IFN-α/β prior to SINV infection, transcriptional responses are generally enhanced, while virus infection is strongly inhibited. However, cellular responses to VEEV infection of primed cells are limited to the initial response to IFN-α/β exposure (i.e., not different from pretreated, mock-infected responses) and have a minimal effect on VEEV replication. Thus, as described above, the expression of viral factors that arrest host macromolecular synthesis may reflect the relative sensitivity to inhibition of replication promoted by the established antiviral state.
In IFN-α/β posttreatment experiments, infection with both viruses either abrogated or diminished upregulation of antiviral gene mRNA synthesis in response to IFN-α/β treatment at both early (6 h p.i.) and late (18 h p.i.) times after infection (Fig. 8). Combined with the data from the previous sections, these results demonstrate that established infection with SINV or VEEV in neurons limits the cellular response to IFN-α/β treatment regardless of whether or not phosphorylation of the STAT pathway components is markedly inhibited.
Structural protein expression is not required for inhibition of STAT1/2 phosphorylation but is differentially required for inhibition of ISG upregulation.
To determine if the sPs and/or nsPs were responsible for STAT1/2 pathway inhibition or the blocking of IFN-α/β-mediated ISG upregulation by the viruses, we infected neurons with SINV-based or VEEV-based replicon particles that expressed the GFP reporter protein instead of the viral structural proteins. In this case, we only analyzed postinfection IFN-α/β treatment effects, since the parental viruses did not block STAT1/2 phosphorylation and did not appear to block ISG upregulation if cells were primed with IFN-α/β prior to infection.
IFN-α/β treatment of cells at 12 or 22 h p.i. after infection with replicon particles recapitulated the inhibitory effects of parental VEEV and SINV virus infection upon phosphorylation of STAT1/2 pathway components, indicating that expression of the nsP and replication of the truncated genome were sufficient and that sP expression was not required (Fig. 9A). As with the parental viruses, replicon infection also resulted in sporadic, minor phosphorylation of STAT1/2 in untreated cells; although, as with the parental viruses, IFN-α/β production in supernatants was not detectable with a biological assay (limit of detection, 3.9 IU/ml). It is likely, however, that this phosphorylation is dependent upon the production of low-level IFN-α/β, as we observed that infection of murine embryo fibroblasts from normal mice resulted in the same sporadic STAT1/2 phosphorylation in the absence of detectable IFN-α/β production while STAT1/2 phosphorylation was not observed when cells from mice lacking a functional IFN-α/β receptor were used (data not shown).
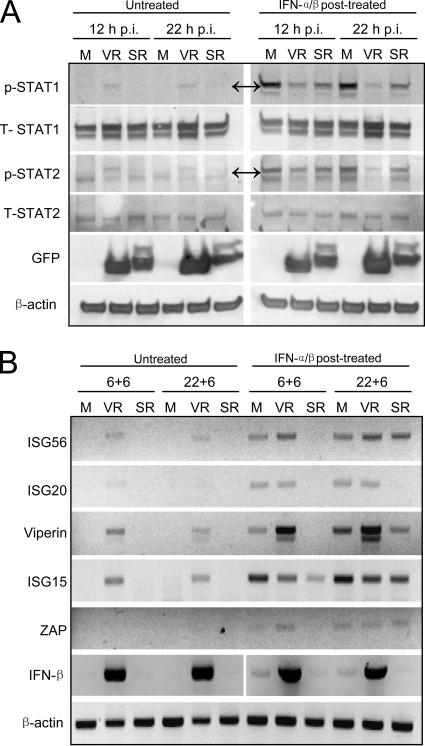
FIG. 9.
Patterns of STAT1 and STAT2 phosphorylation and IFN-β gene and ISG transcription in untreated and IFN-α/β-posttreated neurons infected with VEEV or SINV replicons. Neuron cultures were either mock infected (M) or infected with VEEV or SINV replicons (VR or SR, respectively) as described in Materials and Methods and then either left untreated or treated with 1,000 IU/ml of IFN-α/β. (A) Cells were treated for 30 min at 12 or 22 h p.i. with lysates harvested immediately after IFN-α/β treatment and processed for Western blot for the indicated proteins as described in Materials and Methods. GFP was stained as a control for protein expression from the replicons. In panels with nonspecific staining, double arrows indicate the protein bands of interest. (B) Cells were treated for 6 h beginning at 12 (12 + 6) or 22 (22 + 6) h p.i. and total cellular RNA was harvested 6 h after the onset of IFN treatment and processed for semiquantitative RT-PCR for the indicated mRNAs as described in Materials and Methods. For PCR analyses, IFN-α/β treatment and harvest times were set to allow gene upregulation and minimize cytopathic effects of replicons upon untreated cells.
Consistent with data collected using the parental viruses, RT-PCR analyses indicated that VEEV replicon infection modestly increased the abundance of mRNAs for multiple ISGs and strongly upregulated the IFN-β mRNA in untreated cells (Fig. 9B). However, in contrast with the parental virus IFN-α/β posttreatment results, established VEEV replicon infection had little inhibitory effect on, or actually increased, the abundance of ISG mRNAs following IFN-α/β posttreatment versus uninfected, IFN-α/β-treated cells. The differential effects of VEEV virus and replicon infection most likely reflect that the VEEV capsid protein, previously implicated in shutoff of host gene transcription (21), was not expressed in replicon-infected neurons. Interestingly, the ISG induction results did not correlate with blockade of STAT phosphorylation by the VEEV replicon, which we expected would limit ISG induction after postinfection IFN-α/β treatment. On the other hand, SINV replicon infection did not result in ISG induction in untreated cells and, in most cases, reduced ISG induction versus uninfected cells after IFN-α/β posttreatment, consistent with the parental virus infection and the established role of SINV nsP2 in transcription arrest (20). Together these data indicate that SINV replicons more potently block ISG mRNA upregulation than VEEV replicons in infected neurons independently of effects upon STAT1 phosphorylation. Moreover, the partial inhibition of STAT1 phosphorylation associated with expression of VEEV nsP and replicon genome replication does not correlate well with inhibition of ISG upregulation in primary neurons.
We considered that the effects of STAT phosphorylation blockade upon gene transcription might be more readily discernible in an in vitro system in which cells were not exposed to any IFN-α/β prior to the time at which viral antagonists were fully expressed (e.g., in cells able to respond to exogenously added IFN-α/β but not able to produce any IFN-α/β after virus infection). A murine cell system that responded to but was genetically incapable of IFN-α/β production was not available, so we examined these events after VEEV or SINV replicon infection of primate Vero cells, which exhibit these qualities (37). Like neurons, infection with SINV or VEEV replicons partially blocked STAT1 phosphorylation at 12 or 22 h p.i. in Vero cells (Fig. 10A); however, unlike neurons, IFN-α/β-induced transcription of all ISGs was diminished by established VEEV replicon infection versus uninfected cells (Fig. 10B). This result is consistent with the idea that signaling by low levels of IFN-α/β induced by VEEV replicon infection potentiated ISG induction in the neurons, although differences between murine and primate cells may also be involved.
FIG. 10.
Patterns of STAT1 phosphorylation and IFN-β gene and ISG transcription in untreated or IFN-α/β-posttreated Vero cells infected with VEEV or SINV replicons. Vero cells were infected with SINV or VEEV GFP-expressing replicons at a multiplicity of infection sufficient to infect >95% of cells. (A) As with neuron cultures, at 12 or 22 h p.i., cells were treated with 1,000 IU of IFN-α/β for 30 min and then harvested immediately as described in Materials and Methods for analysis of STAT1 phosphorylation and abundance. In panels with nonspecific staining, double arrows indicate the protein bands of interest. (B) For semiquantitative RT-PCR, cells were mock treated or treated with IFN-α/β as above for 6 h beginning at 6 (6 + 6) or 16 (16 + 6) h p.i. and then harvested for semiquantitative RT-PCR analysis of ISG and β-actin mRNA abundance. For RT-PCR analyses, IFN-α/β treatment and harvest times were set to allow gene upregulation and minimize cytopathic effects of replicons upon untreated cells. PCR primers were designed for primate versions of each gene based upon mRNA sequences in the NCBI database.
Host cell macromolecular synthesis shutoff is involved in the effects of VEEV and SINV upon ISG induction.
The findings presented so far suggest that virus shutoff of host macromolecular synthesis may be an important, possibly dominant, factor in the abrogation of neuronal responses to virus infection, as well as the response to IFN-α/β added after infection is established. Previous studies have indicated that nsP2 of SINV and the capsid protein of New World viruses such as VEEV and EEEV are directly involved in transcriptional shutoff (1, 19-21, 23). The specific viral mediators of translational shutoff are undefined, but may also be encoded in the nsP2 of SINV (17). To determine the effects of host macromolecular shutoff promoted by VEEV versus SINV viruses and the relationship of this phenomenon to ISG upregulation, we radiolabeled newly produced proteins after infection of the neurons with each type of virus and replicons derived from them. With viruses, cells were either untreated or treated with IFN-α/β prior to infection and host translation rates were measured by 2 h of radiolabeling at 12 or 18 h p.i. With replicons, we examined the capacity for translation inhibition in untreated cells at 12 or 18 h p.i. Only preinfection IFN-α/β treatment was examined, as it would not be expected that IFN-α/β treatment after infection would alter overall translation in a manner that would be observable in a total protein synthesis analysis.
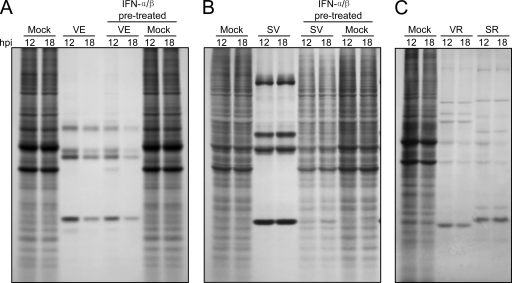
As expected, both SINV and VEEV parental viruses efficiently blocked the accumulation of new host proteins after infection of untreated neurons, with essentially complete shutoff observed by 12 h p.i. (Fig. 11A and B). VEEV also efficiently blocked host protein synthesis after infection of IFN-α/β-pretreated neurons, with a slight delay at 12 h p.i. (Fig. 11A). However, translation inhibition by SINV was greatly diminished in IFN-α/β-pretreated neurons (Fig. 11B), consistent with the greater inhibition of SINV replication and, presumably, reduced expression of viral shutoff mediators after IFN-α/β pretreatment (Fig. 1). With replicons (from which the SINV nsP2 transcriptional inhibitor would be expressed but the VEEV capsid transcription inhibitor would be absent), blockade of accumulation of radiolabeled proteins was observed with both SINV and VEEV in untreated neurons (Fig. 11C).
FIG. 11.
Total protein synthesis in neurons infected with VEEV or SINV or replicons with and without IFN-α/β pretreatment. Neurons were either untreated or treated for 24 h with 1,000 IU/ml of IFN-α/β (A and B) and infected with VEEV (A) or SINV (B) or left untreated and infected with SINV or VEEV replicons (C) as described in Materials and Methods. Cells were labeled with 100 μCi/ml of [35S]Met-Cys for 2 h at 12 or 18 h p.i., and then lysates were made and radiolabeled proteins were separated on SDS-PAGE gels and visualized as described in Materials and Methods.
Overall, when combined with the ISG induction results, these data suggest that VEEV nsP expression arrests translation in virus- or replicon-infected neurons, regardless of whether the cells have been preexposed to IFN-α/β, but sP (presumably capsid) expression is required for transcriptional arrest. In contrast, both SINV and replicon infections potently arrest transcription and translation in untreated cells but do not do so in IFN-α/β-pretreated cells. Furthermore, although infection with both viruses can inhibit phosphorylation of STAT1 and STAT2, this does not appear to preclude ISG induction if neurons are exposed to IFN-α/β prior to infection or if neurons are infected with the VEEV capsid-deleted replicon.
DISCUSSION
Effects of infection upon induction of IFN-α/β.
The results of our studies, and those of other groups, suggest that multiple alphaviruses reduce host cell responses to infection through arrest of macromolecular synthesis (1, 17, 21) (Burke et al., submitted). Accordingly, in the current studies, we were unable to detect released IFN-α/β protein after infection of unprimed primary neurons with SINV or VEEV or replicons. Interestingly, little or no upregulation of IFN-β mRNA was observed in untreated cells infected with SINV or SINV-based replicons, while this mRNA was upregulated after infection with either VEEV or replicons. However, IFN-α/β treatment prior to infection resulted in upregulation of the IFN-β mRNA by SINV to levels similar to those observed after VEEV infection. These data suggest that transcriptional shutoff after SINV infection of unprimed cells is more complete than that after VEEV infection but that IFN-α/β pretreatment limits the ability of SINV to block host transcription. Ultimately, the inhibitory effect upon host translation after infection may account for some of the blockade of IFN-α/β protein production with SINV and the majority of the blockade with VEEV. It should be noted that both viruses induce IFN-α/β after subcutaneous infection of mice (9, 24, 44), implying that other cell types are either more resistant to arrest of host macromolecular synthesis or that IFN-α/β responses arise primarily from uninfected cells in vivo (31). Therefore, it is likely that the capacity of host cells to produce IFN-α/β in response to alphavirus infection is cell type dependent and may be affected by exposure to circulating antiviral cytokines in the infected host.
Effects of infection upon the antiviral state.
Our data indicate that VEEV is significantly more resistant than SINV to the replication-inhibiting activities of the IFN-α/β-induced antiviral state and, furthermore, that both viruses substantially block phosphorylation of STAT1/2 when cells are exposed to IFN-α/β after infection. Other viruses antagonize the response of cells to supernatant IFN-α/β by blocking the JAK/STAT pathway by downregulation of the IFN-α/β receptor, enhancement of degradation rates for pathway components, blockade of their phosphorylation or trafficking, or by induction of activities that result in dephosphorylation (reviewed in references 10 and 43). VEEV and SINV do not appear to enhance JAK/STAT pathway component degradation or dephosphorylation when cells are pretreated with IFN, suggesting that they do not “dismantle” a preexisting antiviral state. The mechanism by which alphaviruses block STAT1/2 phosphorylation could involve direct interaction of viral nsP with IFN-α/β receptor subunits, upstream activators JAK or Tyk, the STAT1/2 kinases themselves, or conceivably, virus-mediated reduction in the abundance of mediators upstream of the STAT1/2 proteins (e.g., cell surface expression of the IFN-α/β receptor).
In cultured neurons, both SINV and VEEV appear to limit ISG expression (as with IFN-β expression described above) in naïve cells and in cells treated with IFN-α/β after infection through shutoff of host macromolecular synthesis. Surprisingly, virus-mediated blockade of STAT1/2 phosphorylation in neurons made only a minor contribution to inhibition of ISG induction in the face of the potent virus-mediated arrest of macromolecular synthesis, even in the absence of VEEV capsid-mediated transcriptional shutoff.
The disconnection between STAT1/2 phosphorylation blockage and inhibition of ISG induction is at least partially explained by the potentiating effect that virus infection had upon ISG induction if cells were exposed to IFN-α/β prior to host macromolecular shutoff. Increased induction of multiple ISGs over IFN-α/β-treated, uninfected controls occurred when cultures were pretreated with IFN-α/β and SINV infected or when VEEV replicon infected and IFN-α/β posttreated. As described above, it is likely that IFN-α/β signaling, either by exogenously added IFN-α/β or by very low levels of IFN-α/β induced in the neurons in response to infection, potentiated ISG induction. This may be due to (i) specific blocking by IFN-α/β signaling of virus-mediated transcriptional shutoff activities, (ii) IFN-α/β-mediated induction of pattern recognition receptors or their downstream signaling partners that stimulate IFN-α/β gene induction (e.g., RIG-I, MDA-5, and IRF-7 are all IFN-α/β inducible [12, 28, 29, 34, 56]), or (iii) increased abundance of IFN-α/β receptor signaling pathway-related molecules, such as the STAT proteins themselves (14). Higher levels of IFN-α/β induction or IFN-α/β receptor signaling pathway components may increase the activity of signaling cascades to the point where inhibition of STAT phosphorylation is overcome. Accordingly, when we removed the possibility of low-level IFN-α/β production in response to VEEV replicon infection by using Vero cells that were are genetically deficient in production of IFN proteins, inhibition of STAT1/2 phosphorylation was correlated with inhibition of ISG upregulation in response to added IFN-α/β.
While our results are inconclusive with respect to the importance of JAK/STAT pathway blockade in cells capable of producing IFN-α/β in response to infection, it is possible that this delays clearance of virus infection in neurons provided that sP-mediated macromolecular shutoff is not highly efficient and the ISG transcription-stimulating effect of IFN-α/β exposure is less prominent. This could reflect a virus-mediated antagonistic effect upon IFN-mediated clearance from neurons such as the noncytolytic clearance of SINV mediated by IFN-γ (which also signals through the STAT1 pathway [14]) released by T cells (4, 7).
Viral proteins responsible for macromolecular shutoff.
Consistent with previous studies utilizing fibroblast cultures (17, 21), we found that the overall arrest in host transcription resulting in suppression of neuron IFN-β and ISG mRNA production was associated with VEEV sP and SINV nsP. While transcription and translation shutoff were not conclusively distinguished in our studies with SINV due to the potential role of nsP in both processes, we unexpectedly found that the VEEV nsP in the context of a replicating genome and in the absence of capsid expression potently arrested translation, but not transcription, in infected neurons. This occurred even when the cells were treated with IFN-α/β prior to infection. This result is in contrast with a limited transcription or translation shutoff after VEEV replicon genome electroporation into BHK 21 fibroblasts reported by Garmashova et al. (21). This may reflect different effects of infection versus electroporation, a strain difference between the parental viruses from which the replicons were derived, or cell-type-specific differences. We found that VEEV replicon infection resulted in only partial shutoff of translation in Vero monkey kidney fibroblast cells (data not shown), and we interpret these results to indicate that the capacity of VEEV nsP to shut off translation is cell type dependent. The fact that the translation shutoff activity of VEEV is resistant to IFN-α/β pretreatment of cells may underlie some of the pathology associated with replication of the virus (9, 13, 26, 27, 53) or replicons (50) in the brain.
Effects of alphavirus infection upon neurons in the infected host.
In nature, alphaviruses are delivered to the host by mosquitoes and interact initially with myeloid cells (18, 35, 45). Subcutaneous infection of mice with VEEV or SINV results in production of IFN-α/β that is secreted into the serum (9, 24, 44, 55). Notably, VEEV infection results in the highest serum IFN-α/β induction of any alphavirus we have tested, including VEEV, SINV, EEEV, and CHIKV (18, 44) (data not shown). Presumably, serum IFN-α/β crosses the blood-brain barrier and activates the JAK/STAT pathway in cells of the central nervous system. Therefore, by the time of virus neuroinvasion, an antiviral state would already be established. While both SINV and VEEV inhibit JAK/STAT signaling in neurons, we propose that the greater neurovirulence of VEEV in vivo is explained, at least in part, by resistance of VEEV replication to the preestablished antiviral state. This resistance may also allow VEEV to arrest macromolecular synthesis in cells exposed to IFN-α/β prior to infection, whereas SINV replication and macromolecular synthesis arrest are largely abrogated.
Mechanisms through which VEEV resists the antiviral state are not clear. In separate experiments we have found that the double-stranded RNA-dependent protein kinase (PKR), an ISG with some antialphavirus activity (48), is much less strongly activated by phosphorylation after VEEV infection compared to SINV infection (data not shown). Whether or not VEEV also avoids or blocks the activity of other antialphavirus proteins, such as p56, ZAP, viperin, ISG20, or ISG15 (3, 33, 57), remains to be determined.
Acknowledgments
We thank DeAquinita McKinney for excellent technical assistance.
This work was supported by National Institutes of Health grants R21 AI069158 (K.D.R.) and R21AI072350 (W.B.K.) and grants from the NIAID, NIH, through the Western Regional Center of Excellence for Biodefense and Emerging Infectious Diseases Research, U54 AI057156 (Career Development award to K.D.R. and major project subcontract to W.B.K.).
Footnotes
Published ahead of print on 29 July 2009.
REFERENCES
- 1.Aguilar, P. V., S. C. Weaver, and C. F. Basler. 2007. Capsid protein of eastern equine encephalitis virus inhibits host cell gene expression. J. Virol. 81:3866-3876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anishchenko, M., S. Paessler, I. P. Greene, P. V. Aguilar, A. S. Carrara, and S. C. Weaver. 2004. Generation and characterization of closely related epizootic and enzootic infectious cDNA clones for studying interferon sensitivity and emergence mechanisms of Venezuelan equine encephalitis virus. J. Virol. 78:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bick, M. J., J. W. Carroll, G. Gao, S. P. Goff, C. M. Rice, and M. R. Macdonald. 2003. Expression of the zinc-finger antiviral protein inhibits alphavirus replication. J. Virol. 77:11555-11562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Binder, G. K., and D. E. Griffin. 2001. Interferon-gamma-mediated site-specific clearance of alphavirus from CNS neurons. Science 293:303-306. [DOI] [PubMed] [Google Scholar]
- 5.Borgherini, G., P. Poubeau, F. Staikowsky, M. Lory, N. Le Moullec, J. P. Becquart, C. Wengling, A. Michault, and F. Paganin. 2007. Outbreak of Chikungunya on Reunion Island: early clinical and laboratory features in 157 adult patients. Clin. Infect. Dis. 44:1401-1407. [DOI] [PubMed] [Google Scholar]
- 6.Boxel-Dezaire, A. H., M. R. Rani, and G. R. Stark. 2006. Complex modulation of cell type-specific signaling in response to type I interferons. Immunity 25:361-372. [DOI] [PubMed] [Google Scholar]
- 7.Burdeinick-Kerr, R., D. Govindarajan, and D. E. Griffin. 2009. Noncytolytic clearance of sindbis virus infection from neurons by gamma interferon is dependent on Jak/STAT signaling. J. Virol. 83:3429-3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Casolari, S., E. Briganti, M. Zanotti, T. Zauli, L. Nicoletti, F. Magurano, C. Fortuna, C. Fiorentini, C. M. Grazia, and G. Rezza. 2008. A fatal case of encephalitis associated with Chikungunya virus infection. Scand. J. Infect. Dis. 40:995-996. [DOI] [PubMed] [Google Scholar]
- 9.Charles, P. C., J. Trgovcich, N. L. Davis, and R. E. Johnston. 2001. Immunopathogenesis and immune modulation of Venezuelan equine encephalitis virus-induced disease in the mouse. Virology 284:190-202. [DOI] [PubMed] [Google Scholar]
- 10.Childs, K. S., J. Andrejeva, R. E. Randall, and S. Goodbourn. 2009. Mechanism of mda-5 inhibition by paramyxovirus V proteins. J. Virol. 83:1465-1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Couderc, T., F. Chretien, C. Schilte, O. Disson, M. Brigitte, F. Guivel-Benhassine, Y. Touret, G. Barau, N. Cayet, I. Schuffenecker, P. Despres, F. Arenzana-Seisdedos, A. Michault, M. L. Albert, and M. Lecuit. 2008. A mouse model for Chikungunya: young age and inefficient type-I interferon signaling are risk factors for severe disease. PLoS Pathog. 4:e29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cui, X. F., T. Imaizumi, H. Yoshida, E. C. Borden, and K. Satoh. 2004. Retinoic acid-inducible gene-I is induced by interferon-gamma and regulates the expression of interferon-gamma stimulated gene 15 in MCF-7 cells. Biochem. Cell Biol. 82:401-405. [DOI] [PubMed] [Google Scholar]
- 13.Dal Canto, M. C., and S. G. Rabinowitz. 1981. Central nervous system demyelination in Venezuelan equine encephalomyelitis infection. J. Neurol. Sci. 49:397-418. [DOI] [PubMed] [Google Scholar]
- 14.Darnell, J. E., Jr., I. M. Kerr, and G. R. Stark. 1994. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science 264:1415-1421. [DOI] [PubMed] [Google Scholar]
- 15.Davis, N. L., L. V. Willis, J. F. Smith, and R. E. Johnston. 1989. In vitro synthesis of infectious venezuelan equine encephalitis virus RNA from a cDNA clone: analysis of a viable deletion mutant. Virology 171:189-204. [DOI] [PubMed] [Google Scholar]
- 16.Fragkoudis, R., L. Breakwell, C. McKimmie, A. Boyd, G. Barry, A. Kohl, A. Merits, and J. K. Fazakerley. 2007. The type I interferon system protects mice from Semliki Forest virus by preventing widespread virus dissemination in extraneural tissues, but does not mediate the restricted replication of avirulent virus in central nervous system neurons. J. Gen. Virol. 88:3373-3384. [DOI] [PubMed] [Google Scholar]
- 17.Frolova, E. I., R. Z. Fayzulin, S. H. Cook, D. E. Griffin, C. M. Rice, and I. Frolov. 2002. Roles of nonstructural protein nsP2 and alpha/beta interferons in determining the outcome of Sindbis virus infection. J. Virol. 76:11254-11264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardner, C. L., C. W. Burke, M. Z. Tesfay, P. J. Glass, W. B. Klimstra, and K. D. Ryman. 2008. Eastern and Venezuelan equine encephalitis viruses differ in their infectivity for dendritic cells and macrophages: the impact of altered cell tropism on pathogenesis. J. Virol. 82:10634-10646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garmashova, N., S. Atasheva, W. Kang, S. C. Weaver, E. Frolova, and I. Frolov. 2007. Analysis of Venezuelan equine encephalitis virus capsid protein function in the inhibition of cellular transcription. J. Virol. 81:13552-13565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garmashova, N., R. Gorchakov, E. Frolova, and I. Frolov. 2006. Sindbis virus nonstructural protein nsP2 is cytotoxic and inhibits cellular transcription. J. Virol. 80:5686-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garmashova, N., R. Gorchakov, E. Volkova, S. Paessler, E. Frolova, and I. Frolov. 2007. The Old World and New World alphaviruses use different virus-specific proteins for induction of transcriptional shutoff. J. Virol. 81:2472-2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gil, M. P., E. Bohn, A. K. O'Guin, C. V. Ramana, B. Levine, G. R. Stark, H. W. Virgin, and R. D. Schreiber. 2001. Biologic consequences of Stat1-independent IFN signaling. Proc. Natl. Acad. Sci. USA 98:6680-6685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gorchakov, R., E. Frolova, and I. Frolov. 2005. Inhibition of transcription and translation in Sindbis virus-infected cells. J. Virol. 79:9397-9409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Griffin, D. E. 1976. Role of the immune response in age-dependent resistance of mice to encephalitis due to Sindbis virus. J. Infect. Dis. 133:456-464. [DOI] [PubMed] [Google Scholar]
- 25.Griffin, D. E. 2001. Alphaviruses, p. 917-962. In D. M. Knipe and P. M. Howley (ed.), Fields virology. Lippincott, Williams & Wilkins, Philadelphia, PA.
- 26.Jackson, A. C., and J. P. Rossiter. 1997. Apoptotic cell death is an important cause of neuronal injury in experimental Venezuelan equine encephalitis virus infection of mice. Acta Neuropathol. (Berlin) 93:349-353. [DOI] [PubMed] [Google Scholar]
- 27.Jackson, A. C., S. K. SenGupta, and J. F. Smith. 1991. Pathogenesis of Venezuelan equine encephalitis virus infection in mice and hamsters. Vet. Pathol. 28:410-418. [DOI] [PubMed] [Google Scholar]
- 28.Jaitin, D. A., and G. Schreiber. 2007. Upregulation of a small subset of genes drives type I interferon-induced antiviral memory. J. Interferon Cytokine Res. 27:653-664. [DOI] [PubMed] [Google Scholar]
- 29.Kang, D. C., R. V. Gopalkrishnan, L. Lin, A. Randolph, K. Valerie, S. Pestka, and P. B. Fisher. 2004. Expression analysis and genomic characterization of human melanoma differentiation associated gene-5, mda-5: a novel type I interferon-responsive apoptosis-inducing gene. Oncogene 23:1789-1800. [DOI] [PubMed] [Google Scholar]
- 30.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Konopka, J. L., L. O. Penalva, J. M. Thompson, L. J. White, C. W. Beard, J. D. Keene, and R. E. Johnston. 2007. A two-phase innate host response to alphavirus infection identified by mRNP-tagging in vivo. PLoS Pathog. 3:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lemant, J., V. Boisson, A. Winer, L. Thibault, H. Andre, F. Tixier, M. Lemercier, E. Antok, M. P. Cresta, P. Grivard, M. Besnard, O. Rollot, F. Favier, M. Huerre, J. L. Campinos, and A. Michault. 2008. Serious acute Chikungunya virus infection requiring intensive care during the Reunion Island outbreak in 2005-2006. Crit. Care Med. 36:2536-2541. [DOI] [PubMed] [Google Scholar]
- 33.Lenschow, D. J., N. V. Giannakopoulos, L. J. Gunn, C. Johnston, A. K. O'Guin, R. E. Schmidt, B. Levine, and H. W. Virgin. 2005. Identification of interferon-stimulated gene 15 as an antiviral molecule during Sindbis virus infection in vivo. J. Virol. 79:13974-13983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu, R., W. C. Au, W. S. Yeow, N. Hageman, and P. M. Pitha. 2000. Regulation of the promoter activity of interferon regulatory factor-7 gene. Activation by interferon snd silencing by hypermethylation. J. Biol. Chem. 275:31805-31812. [DOI] [PubMed] [Google Scholar]
- 35.MacDonald, G. H., and R. E. Johnston. 2000. Role of dendritic cell targeting in Venezuelan equine encephalitis virus pathogenesis. J. Virol. 74:914-922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McCarthy, K. D., and J. de Vellis. 1980. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 85:890-902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosca, J. D., and P. M. Pitha. 1986. Transcriptional and posttranscriptional regulation of exogenous human beta interferon gene in simian cells defective in interferon synthesis. Mol. Cell. Biol. 6:2279-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perri, S., C. E. Greer, K. Thudium, B. Doe, H. Legg, H. Liu, R. E. Romero, Z. Tang, Q. Bin, T. W. Dubensky, Jr., M. Vajdy, G. R. Otten, and J. M. Polo. 2003. An alphavirus replicon particle chimera derived from Venezuelan equine encephalitis and Sindbis viruses is a potent gene-based vaccine delivery vector. J. Virol. 77:10394-10403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pialoux, G., B. A. Gauzere, S. Jaureguiberry, and M. Strobel. 2007. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 7:319-327. [DOI] [PubMed] [Google Scholar]
- 40.Precious, B. L., T. S. Carlos, S. Goodbourn, and R. E. Randall. 2007. Catalytic turnover of STAT1 allows PIV5 to dismantle the interferon-induced anti-viral state of cells. Virology 368:114-121. [DOI] [PubMed] [Google Scholar]
- 41.Pushko, P., M. Bray, G. V. Ludwig, M. Parker, A. Schmaljohn, A. Sanchez, P. B. Jahrling, and J. F. Smith. 2000. Recombinant RNA replicons derived from attenuated Venezuelan equine encephalitis virus protect guinea pigs and mice from Ebola hemorrhagic fever virus. Vaccine 19:142-153. [DOI] [PubMed] [Google Scholar]
- 42.Pushko, P., M. Parker, G. V. Ludwig, N. L. Davis, R. E. Johnston, and J. F. Smith. 1997. Replicon-helper systems from attenuated Venezuelan equine encephalitis virus: expression of heterologous genes in vitro and immunization against heterologous pathogens in vivo. Virology 239:389-401. [DOI] [PubMed] [Google Scholar]
- 43.Randall, R. E., and S. Goodbourn. 2008. Interferons and viruses: an interplay between induction, signalling, antiviral responses and virus countermeasures. J. Gen. Virol. 89:1-47. [DOI] [PubMed] [Google Scholar]
- 44.Ryman, K. D., and W. B. Klimstra. 2008. Host responses to alphavirus infection. Immunol. Rev. 225:27-45. [DOI] [PubMed] [Google Scholar]
- 45.Ryman, K. D., W. B. Klimstra, K. B. Nguyen, C. A. Biron, and R. E. Johnston. 2000. Alpha/beta interferon protects adult mice from fatal Sindbis virus infection and is an important determinant of cell and tissue tropism. J. Virol. 74:3366-3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ryman, K. D., K. C. Meier, C. L. Gardner, and W. B. Klimstra. 2007. Non-pathogenic Sindbis virus causes viral hemorrhagic fever in the absence of alpha/beta and gamma interferons. Virology 368:273-285. [DOI] [PubMed] [Google Scholar]
- 47.Ryman, K. D., K. C. Meier, E. M. Nangle, S. L. Ragsdale, N. L. Korneeva, R. E. Rhoads, M. R. Macdonald, and W. B. Klimstra. 2005. Sindbis virus translation is inhibited by a PKR/RNase L-independent effector induced by alpha/beta interferon priming of dendritic cells. J. Virol. 79:1487-1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ryman, K. D., L. J. White, R. E. Johnston, and W. B. Klimstra. 2002. Effects of PKR/RNase L-dependent and alternative antiviral pathways on alphavirus replication and pathogenesis. Viral Immunol. 15:53-76. [DOI] [PubMed] [Google Scholar]
- 49.Samuel, M. A., K. Whitby, B. C. Keller, A. Marri, W. Barchet, B. R. Williams, R. H. Silverman, M. Gale, Jr., and M. S. Diamond. 2006. PKR and RNase L contribute to protection against lethal West Nile Virus infection by controlling early viral spread in the periphery and replication in neurons. J. Virol. 80:7009-7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schafer, A., A. C. Whitmore, J. L. Konopka, and R. E. Johnston. 2009. Replicon particles of Venezuelan equine encephalitis virus as a reductionist murine model for encephalitis. J. Virol. 83:4275-4286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schindler, C., D. E. Levy, and T. Decker. 2007. JAK-STAT signaling: from interferons to cytokines. J. Biol. Chem. 282:20059-20063. [DOI] [PubMed] [Google Scholar]
- 52.Stark, G. R., I. M. Kerr, B. R. Williams, R. H. Silverman, and R. D. Schreiber. 1998. How cells respond to interferons. Annu. Rev. Biochem. 67:227-264. [DOI] [PubMed] [Google Scholar]
- 53.Steele, K. E., K. J. Davis, K. Stephan, W. Kell, P. Vogel, and M. K. Hart. 1998. Comparative neurovirulence and tissue tropism of wild-type and attenuated strains of Venezuelan equine encephalitis virus administered by aerosol in C3H/HeN and BALB/c mice. Vet. Pathol. 35:386-397. [DOI] [PubMed] [Google Scholar]
- 54.Tesfay, M. Z., J. Yin, C. L. Gardner, M. V. Khoretonenko, N. L. Korneeva, R. E. Rhoads, K. D. Ryman, and W. B. Klimstra. 2008. Alpha/beta interferon inhibits cap-dependent translation of viral but not cellular mRNA by a PKR-independent mechanism. J. Virol. 82:2620-2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trgovcich, J., J. F. Aronson, and R. E. Johnston. 1996. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology 224:73-83. [DOI] [PubMed] [Google Scholar]
- 56.Yoneyama, M., M. Kikuchi, T. Natsukawa, N. Shinobu, T. Imaizumi, M. Miyagishi, K. Taira, S. Akira, and T. Fujita. 2004. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 5:730-737. [DOI] [PubMed] [Google Scholar]
- 57.Zhang, Y., C. W. Burke, K. D. Ryman, and W. B. Klimstra. 2007. Identification and characterization of interferon-induced proteins that inhibit alphavirus replication. J. Virol. 81:11246-11255. [DOI] [PMC free article] [PubMed] [Google Scholar]