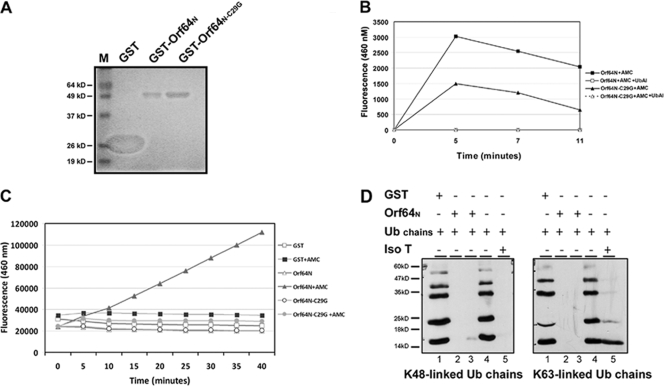
FIG. 3.
The N terminus of Orf64 has deubiquitinating activity for both K48- and K63-linked Ub chains in vitro. (A) GST-Orf64N and GST-Orf64N-C29G were expressed in BL21(DE3) cells with the control GST protein (lane GST). After purification, the proteins were electrophoresed by SDS-PAGE and the gel was stained with Coomassie blue dye. (B) Purified GST-Orf64N (4 μM) was incubated with 2 μM of a wild-type Ub fluorogenic substrate, AMC-Ub. Fluorescence generated from the cleaved AMC-Ub substrate was recorded over time at 460 nm. Data from reactions including AMC-Ub with purified GST-Orf64N-C29G, GST-Orf64N plus Ub-Al, or Orf64N-C29G plus Ub-Al are also shown. (C) AMC-Ub reaction mixtures containing 200 nM of the indicated GST-purified proteins and 1.2 μM AMC-Ub substrate were processed as described for panel B. (D) Purified GST-Orf64N was used in an in vitro DUB assay with Ub chains. Lysine 48 (K48)- and lysine 63 (K63)-linked chains are not cleaved by GST (lanes 1) or in the absence of GST-Orf64N (lanes 4). Ub chains containing K48- and K63-linked Ub moieties were completely cleaved by the positive control, IsoT (lanes 5), as well as in the presence of Orf64N (lanes 2 and 3).