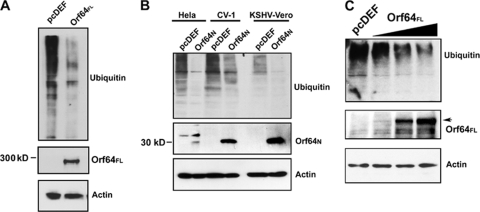
FIG. 4.
Orf64 exerts DUB activity in vivo. (A) Cotransfection of pcDEF3-Orf64FL and HA-tagged Ub expression plasmids in HEK-293 cells. Cells were harvested 48 h posttransfection and subjected to Western blot analysis with an anti-HA antibody. Expression of Orf64FL results in decreased levels of high-molecular-weight ubiquitinated cellular proteins compared to levels seen with the empty vector (pcDEF) control. Western blots for actin show equal loading of protein lysates, and Western blots with anti-Flag antibody show expression of Orf64FL. (B) Cotransfection of pcDEF3-Orf64N and HA-tagged Ub expression plasmids in HeLa, CV-1, and KSHV-positive rKSHV.219-Vero cells (KSHV-Vero). Cells were harvested 48 h posttransfection and subjected to Western blot analysis with anti-HA antibody. Expression of Orf64N results in decreased levels of high-molecular-weight ubiquitinated proteins compared to levels for transfection with the empty vector (pcDEF) control. Thus, the DUB activity is contained within the N-terminal 205 residues of Orf64. Western blots for actin show equal loading of protein lysates, and Western blots with an anti-Flag antibody show expression of Orf64N. (C) Dose-response experiment. 293T cells were transfected with 15 μg of empty vector (lane 1 [left]) or increasing amounts (5, 10, and 15 μg) of Orf64 expression plasmid (lanes 2, 3, and 4, respectively). Cells were harvested 48 hours posttransfection, and lysates were subjected to Western blot analysis with anti-Ub, anti-GAPDH, and antiactin antibodies. There is a decrease in levels of cellular ubiquitinated proteins in the presence of increasing amounts of Orf64FL.