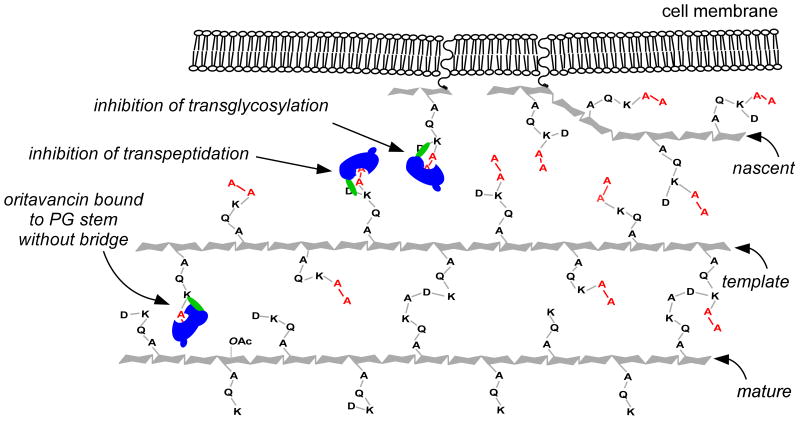
Figure 10.
Two-dimensional schematic representation of the E. faecium template model for peptidoglycan assembly and oritavancin mode of action. Three molecules of bound oritavancin are shown as blue and green icons. D-Ala-D-Ala termini of pentapeptide stems are highlighted in red. The phenylbenzyl moiety of oritavancin is highlighted in green. The middle and right drug icons are bound to both primary- and secondary-binding sites, whereas the left drug icon is only bound to the primary-binding site (D-Ala-D-Ala). Oritavancin has a unique conformation when bound to peptidoglycan stems with both a primary- and secondary-binding site. The secondary-binding site is formed by the triangulation of a D-Asx bridge, an L-Lys residue, and a penultimate D-Ala residue of a pentapeptide stem (middle and right drug icons). Oritavancin primarily inhibits transpeptidase by binding to template and nascent peptidoglycan and sterically interfering with the ability of the D-Asp of one stem to form a cross-link with the penultimate D-Ala of an adjacent stem. Oritavancin assumes a different conformation when bound to a pentapeptide stem without a bridge (these pentapeptide stems have a primary-binding site but do not have a secondary-binding site, see left drug icon). The different confirmation causes the phenylbenzyl moiety of oritavancin to be close to L-Lys, as indicated by the superimposition of the left drug icon over the L-Lys residue. In mature peptidoglycan, there is a high concentration of tripeptides resulting from uncross-linked stems whose D-Ala-D-Ala termini have been cleaved by carboxypeptidase (mature strand). These stems have neither primary- nor secondary-binding sites. While oritavancin can probably inhibit transglycosylation by binding to lipid II (right icon), the drug primarily inhibits transpeptidase because of the larger number of secondary-binding sites associated with the long glycan chains of E. faecium (template strand).