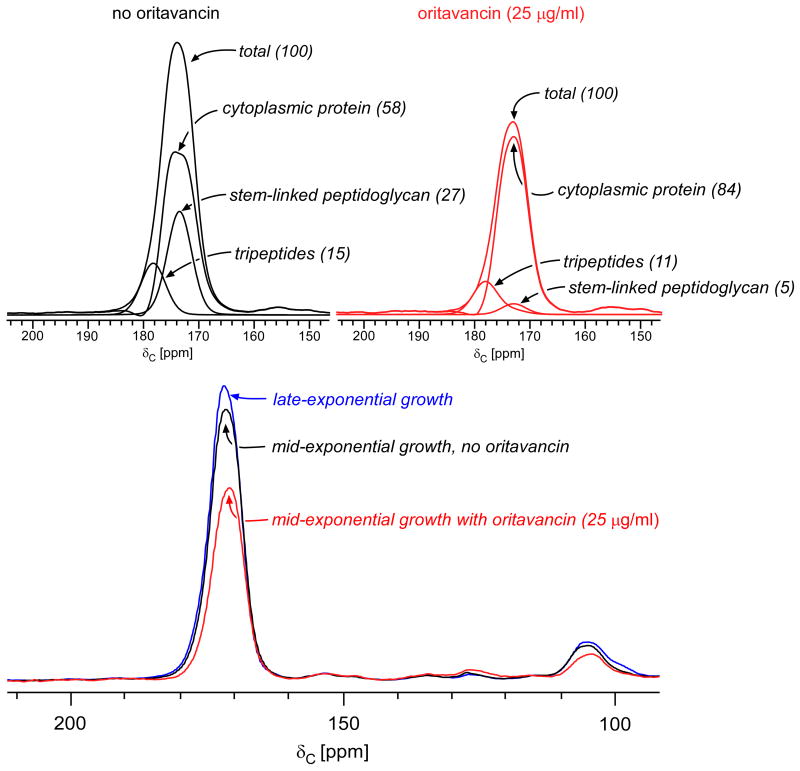
Figure 8.
(Bottom) 13C CPMAS echo spectra for E. faecium whole cells enriched with L-[1-13C]lysine harvested at late-exponential growth (blue), mid-exponential growth (black), and mid-exponential growth in the presence of 25 μg/ml oritavancin (red). The spectra are normalized with respect to the natural-abundance, aliphatic-carbon signal intensities between 0 and 35 ppm. The cytoplasmic protein contribution to the peak centered about 175 ppm remains essentially unchanged. (Top) Deconvolution of the carbonyl-carbon peak for cells in mid-exponential growth, with and without oritavancin. The terminal carboxyl L-Lys in tripeptide peptidoglycan stems has a unique chemical shift of 178 ppm. The remaining peptidoglycan contribution to the peak centered at 175 ppm results from L-Lys stem-linked to D-Ala and was determined by subtracting the contribution of the cytoplasmic proteins and tripeptides from the total. Tripeptides constitute 37% of the peptidoglycan in the untreated control culture (left) and 69% of the peptidoglycan in cells treated with 25 μg/ml of oritavancin (right). The tripeptide concentration increases in cells treated with oritavancin as a consequence of transpeptidase inhibition, resulting in uncross-linked pentapeptides in the cell wall that are cleaved into tripeptides by L,D-carboxypeptidase.