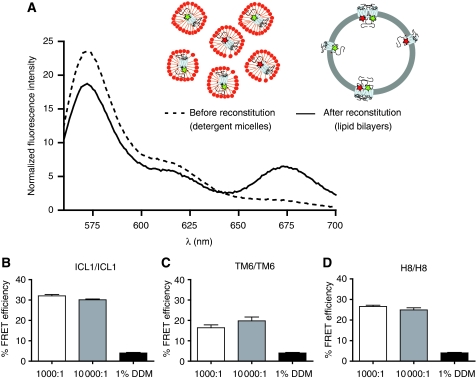
Figure 5.
Intermolecular FRET between Cy3- and Cy5-labelled β2AR is independent of other cellular proteins and is specific. (A) Purified, detergent-solubilized receptor protein was labelled with Cy3 or Cy5 maleimide and unreacted fluorophore was quenched with cysteine and separated from protein by gel filtration as described under Materials and methods. Cy3- and Cy5-labelled protein samples were mixed at a 1:1 molar ratio and reconstituted into phospholipids bilayers or maintained in detergent. Subtraction of the proper controls and normalization of the raw traces is described in the Supplementary data. Labelled β2ARs were reconstituted at a 10-fold higher lipid-to-receptor ratio (10 000:1) and FRET efficiency was measured for ICL1/ICL1 (B), TM6/TM6 (C) and H8/H8 (D) interactions. Data are representative of at least three independent experiments (A) or represent the mean±s.e.m. of at least three independent experiments (B–D).