Abstract
That adult stem cells live in a highly specialized complex microenvironment, also known as a niche, is a pedestrian concept about 30 years old. It may, however, represent a relatively novel approach to being able to modify either normal or abnormal stem cells. Our emphasis in the past has been focused on identifying autonomous regulators of the stem cells and in attempting to modify them through the use of exogenous agents like cytokines. The body modulates these cells largely through the complex system that is embodied in the niche. This report discusses studies in which the niche components are modified to observe their effect on stem cells. The niches being investigated lie in the gut, skin, brain and bone. Other sites for hematopoiesis exist in the body, but these specific microenvironments can be localized and each component can be carefully evaluated using mouse models. Studies are ongoing as to how the stem cell microenvironment can support or propagate malignancies. By understanding the signals of this particular microenvironment, we may be able to adapt them to achieve a therapeutic benefit.
Keywords: leukemia, stem cells, stem cell niches, microenvironment
Introduction
Although the concept of a niche is an old one, it is an idea that has received a fair amount of attention recently. In English, a niche may be defined as an alcove, a cubby-hole, or a recess. In French, the word niche is used as a term to refer to the doghouse, appropriately putting it in its humble place. The niche concept in science is one that is variably defined. From the perspective of environmental science, the niche really is a microenvironment in which the organism or the entity not only resides, but is regulated and nurtured. This comparison can be drawn for stem cells because they live, are regulated, and nurtured in a stem cell-specific microenvironment. The niche is also the place where the stem cell reproduces or self-renews. Thus, the stem cell niche is an anatomically defined space where molecular interactions guide spatial relationships. The niche balances sustaining and constraining elements to tightly regulate stem cell number and function and has the ability to modulate these cells under conditions of physiologic change. The stem cell interactions that occur with the heterologous units that make up the anatomically defined space are guided by very specific molecular interactions; if modified they can change the relationship of the stem cell with both its niche as well as altered state. A set of dynamic balancing cues define stem cell fate and can ultimately modulate the biology of the niche by altering the conditions in which the stem cell resides.
Information on the concept of the niche has been derived from invertebrate models of Drosophila through the work of Alan Spradling and Margaret Fuller1,2 and of Caenorhabditis elegans (C elegans) through the work of Judith Kimble.3 These studies revealed that the function of stem cells in these organisms is highly regulated by the specific structures that house them. For example, in Drosophila and C elegans, the germ stem cells are found at the distal tip of a tapered ovariole structure. The physical interactions between the stem cell and the somatic cells at the end of the structure are essential for the survival of the stem cell. These studies have shown that the number of stem cells is tightly regulated, that the physical association of the niche with the stem cells is essential, and that physical orientation can affect the symmetry of cell division. For example, the orientation of the cell, ie, the relative polarity of the cell, is critical in dictating the outcome of a cell division. It could divide symmetrically, where both daughter cells are identical stem cells, or asymmetrically, so that one daughter cell is more differentiated. Polarity in the relationship of the stem cell to the niche and the orientation of the niche itself seem to be critical determinants of symmetry versus asymmetry. Thus, the niche imposes upon the stem cell its cell fate, which is dictated by the cues that are generated from the niche.4
Stem cells and disease
How does this relate to the development of malignancy? The stem cell state is often imposed through inhibition of differentiation, vacant niches can be viably sustained, ectopic cell occupancy of the niche can result in proliferation of the ectopic cell and more mature descendent cells can revert to a stem cell phenotype if they engage a vacant niche. These findings have been reported by Alan Spradling and his colleagues, and are important in providing rationale to determine how the niche may participate in disease. For example, if the stem cells are removed from this microenvironment, they cease to be viable, but the niche itself retains its viability. If the niche is vacant, there may be other heterologous cell types, including cells that actually make up some of the niche components that can then move into position to engage the distal area of the niche; these cells can then undergo different phenotypic changes including a non-proliferating somatic cell becoming proliferative. In another experimental setting, a more mature population of cells may occupy a vacated niche and undergo a reversion to a stem cell phenotype. The niche can impose a change in differentiation status of its occupants.4
The theory that unoccupied niches in other systems may be able to revert a population of cells to a more undifferentiated state or impose upon the cells a proliferative stimulus could explain how the niche may contribute to disease, particualry neoplastic disease. These findings have only been shown in invertebrates, and there is not enough knowledge about the mammalian stem cell niches to be able to determine whether they apply to mammalian systems. However, they provide intriguing models that need to be explored.
Relatively few stem cell niches—ones in the brain, skin, gut, and bone marrow—have been defined in mammals. In the nervous system, some information is available about where primitive cells reside and the cells that are associated with them in the paraventricular zone, the endothelium.5 The anatomical localization of stem cells within the skin and their relationship with the mesenchymal cells that make up the dermal papillae is necessary for the outcome or fate of the stem cell. So a mesenchymal cell–stem cell interaction is a definitive determinant as to what their fate will be.6,7 In the gut, ephrins can pattern the domain of the niche, and if the niche is disrupted in its anatomic organization by changing ephrin signaling, aberrant proliferation of stem cells can result in tumors.8 In the hematopoietic system, the complexity of the niches that are present in the marrow space is being discovered. It has long been recognized that bone marrow must be in bone for reasons other than just a convenience, but the specific nature of what bone does for bone marrow has been relatively recently defined. The anatomic structure within the marrow space has been appreciated by morphologists for some time.9 The discovery that some stem cells reside in close proximity to endosteum derived from studies where stem cells could be isolated, labeled, then reintroduced into their original environment. These studies found that the stem cells generally resided within six cell diameters of the endosteum surface when transplanted.10 It is relevant to note that the endosteum surface is not the only niche for stem cells. In the mouse, the spleen can serve as a hematopoietic resource for the organism. Hematopoiesis does not occur in places like the spleen or lymph node under normal circumstances humans, but there must be changes in these sites that enable them to become niches in settings such as myelofibrosis where extramedullary hematopoiesis emerges.
Recent studies suggest that there may be more than one type of niche within the marrow space. One study found that cell surface receptors of the signaling lymphocytic activation molecule (SLAM) family, including CD150, CD244, and CD48, are differentially expressed on hematopoietic cells and are markers of stem cells. A stem cell was identified as CD150(+)CD244(-)CD48(-) cells, ie, expressing only the CD150 antigen. It was a rare cell type and found to be associated with vascular structures. The concept of a perivascular niche is now gaining some momentum; however, there is not yet clear evidence of a functional niche in the perivascular space.11 It remains formally possible that the collection of cells in the perivascular region is simply a stopping point en route to or from the circulation and may be regulated there.
The niche: Regulation of stem cells
A number of studies have been conducted to understand the components of the edosteal niche where the stem cells reside. In my lab, we have considered three major categories that distinguish the bone from other types of mesenchymal tissue: mesenchymal cells, extracellular matrix and mineral content. Genetic models have been used to modify each of these different components as a way to identify the regulatory parts of the bone that make an impact on stem cell behavior. One model we used was mice engineered to produce osteoblast-specific, activated parathyroid hormone receptors (PTH/PTHrP). The osteoblast was modified by introducing the constitutively active PTH/PTHrP gene with an osteoblast-specific promoter. Under these conditions stem cells were increased along with osteoblasts, and the osteoblast produced high levels of the Notch1 ligand, Jagged1. Inhibition of Notch1 by gamma secretase abrogated the observed increase of stem cells. PTH-receptor-stimulated osteoblastic cells also support an increase in the number of haematopoietic stem cells with evidence of Notch1 activation in vivo. Notch1 activation may be the basis for activated osteoblasts increasing the number of stem cells (Figure 1).12
Figure 1.
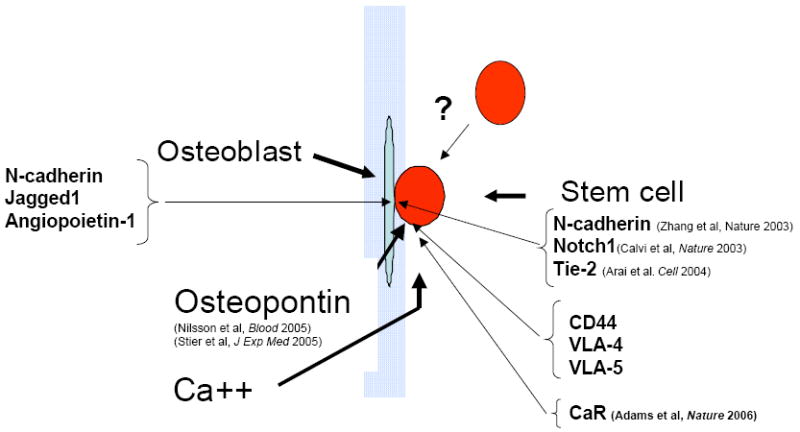
Interactions in the endosteal niche.
This cartoon represents the functional elements of the endosteal niche. It remains controversial as to whether the stem cell resides in immediate contact with osteoblasts or whether the endosteal surface is a more important site of location than the perivascular regions in the bone marrow. However, functional studies have indicated that certain elements of bone are important in regulating stem cells as shown here.
This hypothesis was also approached in Linheng Li’s laboratory using signals generated from bone morphogenetic protein (BMP) to identify its role in the bone: blood interaction. Mutant mice with conditional knockout of BMP receptor type IA (BMPRIA) had an increase in the number of osteoblasts, an accompanying increase in the number of stem cells, and a homotypic interaction of N-cadherin at the interface between stem cells and cells lining the bone surface were thought to mediate the interaction.13
A subsequent study by Toshio Suda’s lab has shown that Tie-2 and angiopoietin-1 are also important at the interface. Angiopoietin-1 produced by osteoblasts activates Tie-2 on stem cells and promotes tight adhesion of these cells to the niche, resulting in quiescence and enhanced survival of these cells. Many other molecules have been suggested as being present, but there is a paucity of anatomic detail (see Figure 1).14
With respect to the relationship of marrow to bone, some studies suggested that if the femoral space of a mouse were either mechanically or chemically disrupted, there might be reconstitution of the stem cell compartment from the cells lining the Haversian and Volkmann canals in bone. These lining cells were somehow the last resort for the reconstitution of hematopoiesis. Studies by Paul Simmons have suggested that the mesenchymal stem cells reside in perivascular space and have the capacity to differentiate into osteoblasts. There may be ongoing interaction between marrow and bone and simple models of hematopoietic stem cells engaging endosteal osteoblasts likely greatly oversimplify a complex integration of two tissue types.15
Role of extracellular matrix components
The extracellular components of the marrow space also appear to important for regulating stem cells. In particular the glycoprotein osteopontin, which is abundant in bone and has been shown to have important functions in the immune system, such as regulating T cells and monocytes. In an osteopontin-null mouse model, the absence of osteopontin was sufficient to increase the number of stem cells. This was associated with increased stromal Jagged1 and angiopoietin-1 expression and reduced primitive hematopoietic cell apoptosis.16 These studies were corroborated at the same time using a similar model in the Nilsson laboratory.9 Osteopontin is capable of interacting with a number of different surface molecules that are on stem cells, including CD44, the β1 integrins, and very late antigen (VLA)-4 and VLA-5. These molecules are interesting because blocking the interaction of osteopontin with stem cells increases their expression. In the absence of osteopontin, if the osteoblast is stimulated, a super physiologic increase in stem cells is observed. Osteopontin is one of the constraining factors that limits the ability of the stem cell to proliferate and provides, in some ways, a balance on stem cell productivity that the niche can accomplish (see Figure 1).
Does the unique mineral content of bone contribute to the localization of stem cells? There is data about the increase and the concentration of ionic calcium immediately surrounding the region where osteoblasts and osteoclasts actively remodel bone; this gradient can be quite substantial. We hypothesized that the stem cell may be able to recognize that extracellular calcium content. The calcium-sensing receptor (CaR)17 is a seven membrane spanning receptor that does not serve as a channel to transport calcium, but recognizes extracellular calcium. In a null mouse model, in the absence of this receptor, the stem cells were unable to engraft at the endosteal surface in a competitive setting (see Figure 1).18 Based on these cumulative findings, we are beginning to understand some of the intricacy of the interactions between bone and bone marrow. Some of these points of interaction may be targeted for therapy to alter stem cell outcomes.
What is particularly attractive about some of the molecules discussed above is that there are drugs that have been developed for other diseases that may be applied to alter the bone: bone marrow interface. One of these, teriparatide, manufactured by Eli Lilly and marketed under the brand name Forteo®, is commercially available. Two others are being tested clinically. Natalizumab (Tysabri,® Biogen Idec) has been approved for multiple sclerosis and cinacalcet (Sensipar,® Amgen) is on the market for people with renal failure-associated hypercalcemia. Natalizumab affects CD44, VLA-4, and VLA-5 adhesion interactions. Ostabolin-C (Zelos Therapeutics) also modulates osteoblasts, and cinacalcet modulates calcium receptors. Agents that modulate the interaction between stem cell and the niche will allow us to address the question whether modifying the niche can impact the outcome of the relevant stem cell.
Modifying the niche to affect normal stem cells
This hypothesis was tested by examining the impact of PTH on bone marrow transplantation. Under homeostatic conditions the number of stem cells could be modified only by about two-fold, hence conditions of stress were used to obtain more exaggerated response. In the model with a genetically active PTH receptor, the wild-type animals were treated with a cognate ligand, PTH. The animals were irradiated and engrafted with an inadequate number of stem cells, a number that would not fully engraft all the animals, ie, about 2- to 3-fold too few to gain 100% engraftment. They were then treated with either PTH or saline and the marrow histology at 2 different time points was examined. What was observed was that marrow cellularity was markedly increased if the animals were treated with PTH compared to saline control after infusion of stem cells. This translated into a fairly dramatic change in overall survival (Figure 2). The stress on the stem cell pool could have led to a premature depletion, but that was not the case. There was a sustained increase in the number of stem cells when these animals were secondarily transplanted.
Figure 2.
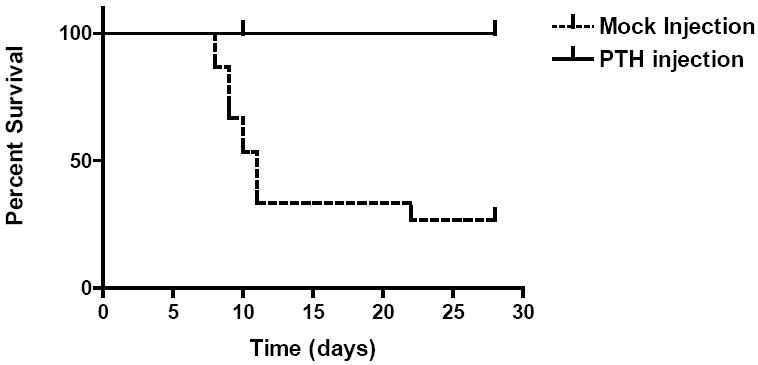
Impact of PTH on bone marrow transplantation. In a mouse model of parathyroid hormone (PTH) receptor activation, transplanted mice stimulated with PTH show improved survival of stem cells over saline injected mice.12
These studies have led to the development of a clinical study by Ballen and colleagues that has now received IRB approval and FDA approval and is enrolling patients.19 In this study, the hypothesis being tested is that it may be possible to use PTH as a way to augment the efficiency of engraftment by modifying the niche. This study is a double cord blood transplant with or without PTH. It is relevant to note that PTH receptors are not present on stem cells. The concept is to modify the receptivity of the niche itself as a way to augment the efficiency of engraftment, and in that way possibly change the outcome of cord blood transplant.
Another issue of interest is whether or not PTH could be used to protect stem cells in an environment that resembles a patient receiving an autologous transplant. Hence, the animal was exposed to sequential doses of cyclophosphamide, followed by either PTH alone, G-CSF alone, the combination of the two, or saline as a control. We measured the number of stem cells that were still resident in the marrow after the sequential stress, and also whether the stem cells could be mobilized into the peripheral blood. The number of stem cells was monitored through a competitive transplant model where the stem cells from PTH-treated animals were transplanted into lethally irradiated animals. The results showed that there was an improvement in the number of stem cells if the animals received PTH versus G-CSF versus the combination. We found that if PTH was given even without G-CSF support, there was still an increase in the number of stem cells in these animals. If the animals received G-CSF support and PTH was also administered, they had a preservation of stem cells above that of G-CSF alone in both the blood and bone marrow. These results showed that there is a mechanism by which PTH is capable of not just increasing the number of stem cells under homeostatic conditions, but also potentially protecting stem cells under conditions of cytotoxic stress.
Currently, there is an ongoing phase I clinical study chaired by Karen Ballen with patients who are undergoing autologous transplant. The patients enrolled in the study are those who have failed their first pheresis. This group is only receiving PTH for a 2-week interval, which is too short for a mouse, and so we suspect it will be too short for a human as well. The testing doses are a two to five-fold increase over that which is used in humans for the treatment of osteoporosis. The reason behind this short interval is because a change in the overall plan of action for these patients was disallowed. The study will then truly only be useful as a safety study and preliminary data appear encouraging in that regard.
Modifying the niche to affect malignant cells
Another question is that if the niche can be modified to change the level of normal stem cells, is there a possibility of being able to use the niche to target malignant cells. The cancer stem cell hypothesis suggests that tumor cells are heterogeneous, but most cells can proliferate and form new tumors; or alternatively, tumor cells are heterogeneous and only the cancer stem cell subset has the ability to proliferate and form new tumors.20 If the cancer stem cell concept holds, then there are other concepts of tissue organization that may also hold. If there is a stem cell pool in malignant tissue in the same way there is in normal tissue, then this pool may also be dependent on a specialized niche. The relationship of this malignant stem cell with its niche may be potentially alterable by the kinds of approaches that have been used for normal stem cells. So the question being tested is if a malignant cell competes with a normal cell for the same niche, can the niche be modified so as to alter the relationship between the niche and these two cell types.
Two questions need to be tested; one is whether the leukemic stem cell occupies the same niche as the normal stem cell, and the other is whether the niche modifying agent can affect leukemic stem cells in the same way it does the normal cells. Leukemic cells were isolated, labeled ex vivo, and transplanted into an animal. Their localization into the bone could be observed with microscopy. A photomicrograph illustrating the margin of the bone and the endosteal surfaces and the bony trabeculae shows that the leukemic cells labeled ex vivo with green fluorescent protein (GFP) that have been restained in the sections show that they have migrated toward the perioendosteal region, comparable to what is seen with a normal stem cell.
The second question to assess the relative effect of niche stimulation in leukemic cells and normal cells was answered by using a C57/black6 mouse with a spontaneous leukemia. This mouse is cultivated as a line, C1498. The leukemic cells were isolated from this C57/black 6 mouse and labeled using retrovirally introduced GFP. Varying ratios of normal cells that were derived from the syngeneic animal to that of leukemia cells were transplanted into irradiated (10 Gy) recipients with PTH or with saline control. In 14-day intervals, the animals were evaluated for engraftment. Measuring the levels of GFP by FACS (fluorescence assisted cell sorting), there was indeed a difference in the marrow populations between animals that had been treated with saline or PTH in the overall ability of the cells to engraft during this 14-day period. In this preliminary data with 5 animals in each arm using saline as the control, there appears to be two populations of labeled cells, whereas with PTH there is a single population of labeled cells, indicating that it may be possible to modify the ratio of the normal versus leukemic cell. The cells themselves do not have the receptor for PTH and are not stimulated by PTH, but this is an effect that is presumably induced by the niche. This experiment raises questions about the possible mechanisms behind this finding. These cells could have a distinctive niche, and that by giving PTH, only one niche expands and not the other. If that were the case, there would be a relative shift in the number of stem cells with the preservation of this niche and the cells would still be able to engraft. The absolute number of malignant cells engrafting would be about the same. However, that was not the case. There was a decrease in the absolute number as well as the relative number of stem cells. The second theory was a change in the ability of the cells to find the niche. There may be receptors that affect the relative efficiency with which a normal stem cell responds to signals of migration generated by PTH, whereas the leukemic cell may not recognize those signals and therefore they may not arrive at the niche or lose the contest with normal cells to home to the niche. Homing studies looking at the ability of cells to find their way to the marrow space showed that homing is unaffected by PTH. Our conclusions are that there may be differing sensitivities between normal versus leukemic cells to signals that are derived from the niche, which are augmented with PTH treatment. This may occur because there may be either an inhibitory signal towards the malignant cell or a stimulatory signal towards the normal cell that shifts this relative balance. In order to evaluate this phenomenon, this system will be moved ex vivo. Osteoblasts (CD51+ and CD45- adherent cells) can be isolated and cultivated ex vivo and the particular molecular mechanisms that may control the differences in normal versus malignant cells moving into the niche may be determined.
The working model of the stem cell–niche interaction is one that can be modified by niche-specific agents. The relative balance between occupation of the niche by normal versus leukemic cells can be altered by modifying the niche. Changing the relative support for the normal cell over the leukemic cell may ultimately be beneficial for the survival of the normal cell. Differing sensitivity to PTH-induced signals is necessary for engraftment and growth of the normal cell.
The animal model used in these studies was a highly artificial one, but data generated by Lilly Pharmaceuticals relate to this subject. When Lilly was testing toxicity of PTH in their animal models they found that there was a dose-related decrease in the incidence of spontaneous leukemia development in Fischer rats treated with PTH. These animals get NK- type leukemia and the idea that PTH could have an inhibitory effect on the development of a spontaneous leukemia was quite intriguing. This was also observed by a Japanese group.21
Conclusion
We occasionally observe after autotransplantation or allotransplantation that patients do not recover their stem cells despite receiving high levels of CD34+ progenitor cells. In animal models, the ability of the animals to recover their full complement of stem cells after a transplant is compromised. The presumed basis for this is that the preparation regimen has in some way affected the niche, so it no longer has the same nurturing capability. Additionally, these studies raise the issue that under transplant conditions, there may be agents that rather than drive hematopoiesis, might affect the osteoblast component. Understanding the niche has ramifications beyond simple biologic interest. Niche biology and function has relevance not only in bone marrow transplantation, but in developing agents that may impact on the ability to generate a larger number of stem cells or increase the efficiency of stem cells to engraft in the transplant setting. These studies can also aid in potentially modifying the relative abundance of normal versus malignant cells in the context of the postchemotherapy setting in AML or MDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Xie T, Spradling AC. A niche maintaining germ line stem cells in the Drosophila ovary. Science. 2000;290:328–330. doi: 10.1126/science.290.5490.328. [DOI] [PubMed] [Google Scholar]
- 2.Kiger AA, White-Cooper H, Fuller MT. Somatic support cells restrict germline stem cell self-renewal and promote differentiation. Nature. 2000;407:750–754. doi: 10.1038/35037606. [DOI] [PubMed] [Google Scholar]
- 3.Crittenden SL, Bernstein DS, Bachorik JL, Thompson BE, Gallegos M, Petcherski AG, Moulder G, Barstead R, Wickens M, Kimble J. A conserved RNA-binding protein controls germline stem cells in Caenorhabditis elegans. Nature. 2002;417:660–663. doi: 10.1038/nature754. [DOI] [PubMed] [Google Scholar]
- 4.Spradling A, Drummond-Barbosa D, Kai T. Stem cells find their niche. Nature. 2001;414:98–104. doi: 10.1038/35102160. [DOI] [PubMed] [Google Scholar]
- 5.Udani VM. The continuum of stem cell transdifferentiation: possibility of hematopoietic stem cell plasticity with concurrent CD45 expression. Stem Cells Dev. 2006;15:1–3. doi: 10.1089/scd.2006.15.1. [DOI] [PubMed] [Google Scholar]
- 6.Jones PH, Watt FM. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell. 1993;73:713–724. doi: 10.1016/0092-8674(93)90251-k. [DOI] [PubMed] [Google Scholar]
- 7.Jensen UB, Lowell S, Watt FM. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development. 1999;126:2409–2418. doi: 10.1242/dev.126.11.2409. [DOI] [PubMed] [Google Scholar]
- 8.Ormestad M, Astorga J, Landgren H, Wang T, Johansson BR, Miura N, Carlsson P. Foxf1 and Foxf2 control murine gut development by limiting mesenchymal Wnt signaling and promoting extracellular matrix production. Development. 2006;133:833–843. doi: 10.1242/dev.02252. [DOI] [PubMed] [Google Scholar]
- 9.Nilsson SK, Johnston HM, Whitty GA, Williams B, Webb RJ, Denhardt DT, Bertoncello I, Bendall LJ, Simmons PJ, Haylock DN. Osteopontin, a key component of the hematopoietic stem cell niche and regulator of primitive hematopoietic progenitor cells. Blood. 2005;106:1232–1239. doi: 10.1182/blood-2004-11-4422. [DOI] [PubMed] [Google Scholar]
- 10.Nilsson SK, Dooner MS, Tiarks CY, Weier HU, Quesenberry PJ. Potential and distribution of transplanted hematopoietic stem cells in a nonablated mouse model. Blood. 1997;89:4013–4020. [PubMed] [Google Scholar]
- 11.Kiel MJ, Yilmaz OH, Iwashita T, Yilmaz OH, Terhorst C, Morrison SJ. SLAM family receptors distinguish hematopoietic stem and progenitor cells and reveal endothelial niches for stem cells. Cell. 2005;121:1109–1121. doi: 10.1016/j.cell.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 12.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–846. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 13.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–841. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 14.Arai F, Hirao A, Suda T. Regulation of hematopoietic stem cells by the niche. Trends Cardiovasc Med. 2005;15:75–79. doi: 10.1016/j.tcm.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116:1827–1835. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 16.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–1791. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNeil SE, Hobson SA, Nipper V, Rodland KD. Functional calcium-sensing receptors in rat fibroblasts are required for activation of SRC kinase and mitogen-activated protein kinase in response to extracellular calcium. J Biol Chem. 1998;273:1114–1120. doi: 10.1074/jbc.273.2.1114. [DOI] [PubMed] [Google Scholar]
- 18.Adams GB, Chabner KT, Alley IR, Olson DP, Szczepiorkowski ZM, Poznansky MC, Kos CH, Pollak MR, Brown EM, Scadden DT. Stem cell engraftment at the endosteal niche is specified by the calcium-sensing receptor. Nature. 2006;439:599–603. doi: 10.1038/nature04247. [DOI] [PubMed] [Google Scholar]
- 19.Ballen KB, Shpall EJ, Avigan D, Yeap B, McAfee S, Dey RD, Attar E, Kronenberg H, Antin JH, Spitzer TR. Parathyroid hormone may improve autologous stem cell mobilization va the stem cell niche. Blood. 2005;106:557a. abstr. [Google Scholar]
- 20.Reya T, Morrison SJ, Clarke MF, Weissman IL. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 21.Onuma E, Tsunenari T, Saito H, Sato K, Yamada-Okabe H, Ogata E. Parathyroid hormone-related protein (PTHrP) as a causative factor of cancer-associated wasting: possible involvement of PTHrP in the repression of locomotor activity in rats bearing human tumor xenografts. Int J Cancer. 2005;116:471–478. doi: 10.1002/ijc.21038. [DOI] [PubMed] [Google Scholar]


