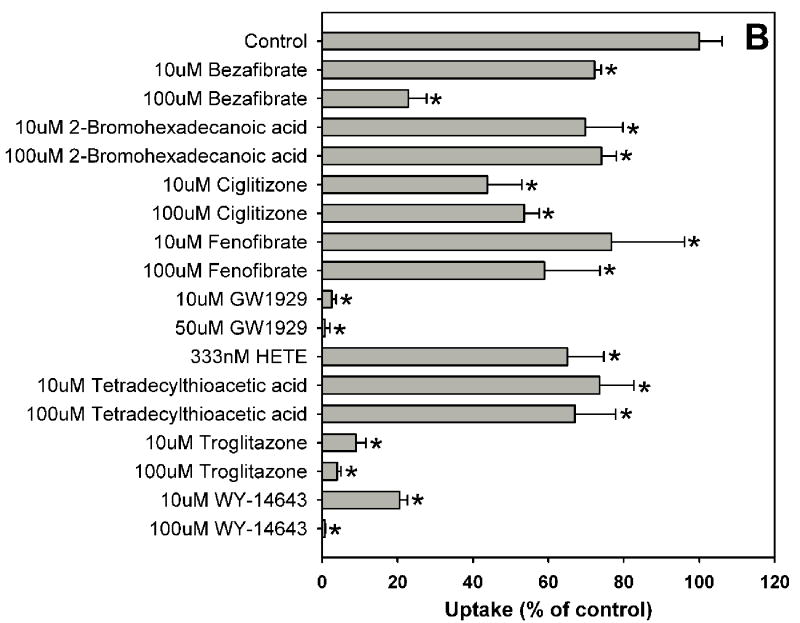
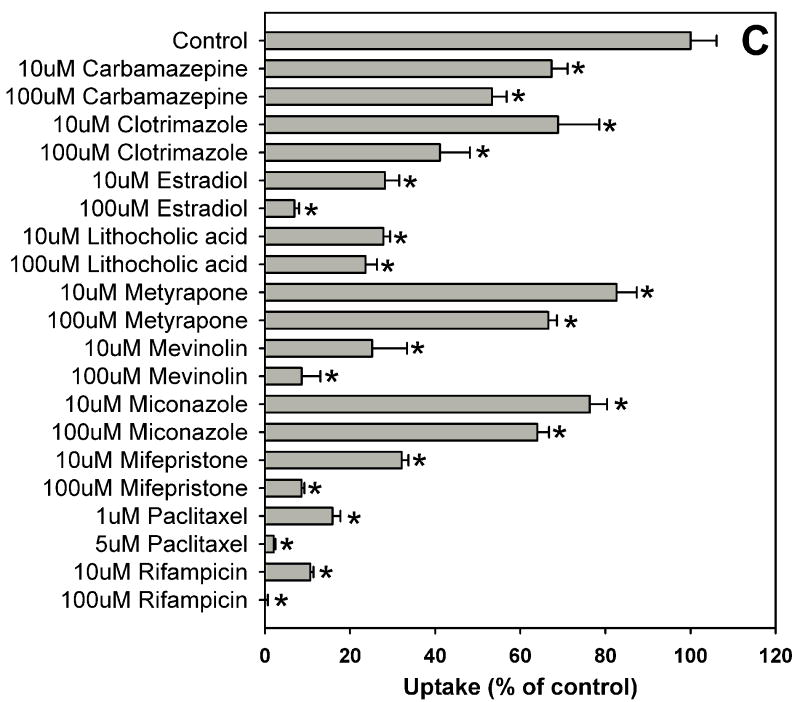
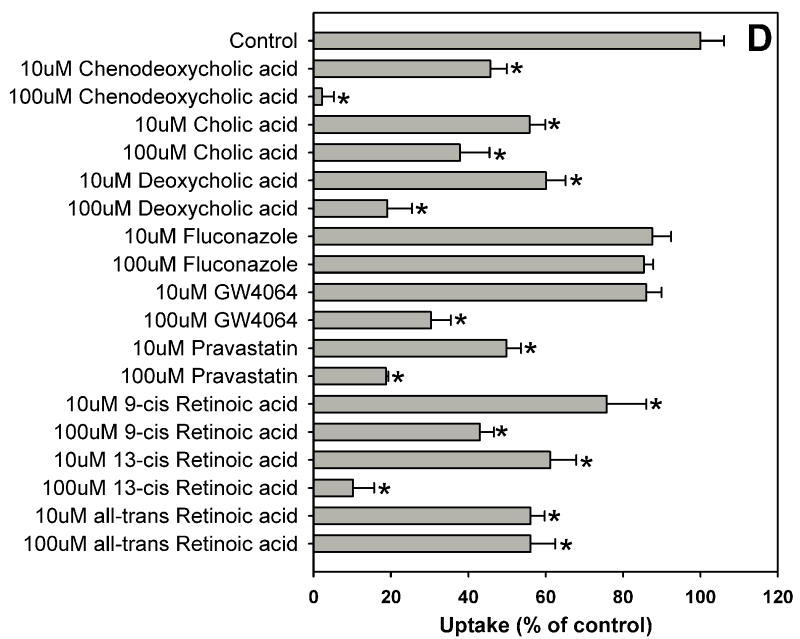
Fig. 1.
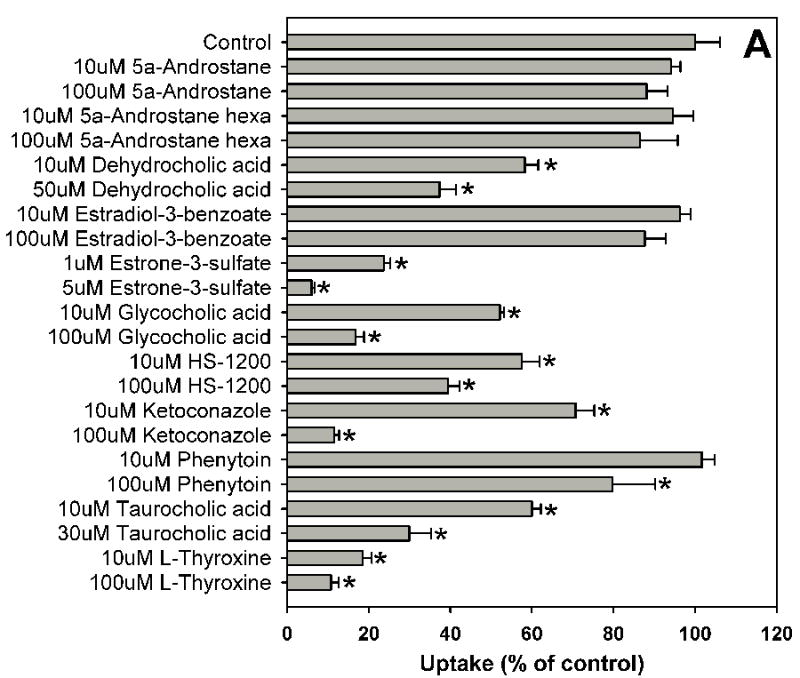
Inhibition of OATP1B1-mediated estradiol-17β-glucuronide uptake by 37 nuclear receptor ligands, the HMG-CoA reductase inhibitor pravastatin and the anti-fungal agent fluconazole. (A) Androgen receptor (AR), constitutive androstane receptor (CAR) and glucocorticoid receptor (GR) ligands. (B) Peroxisome proliferator-activated receptor (PPAR) ligands. (C) Pregnane X receptor (PXR) ligands. (D) Farnesoid X receptor (FXR), retinoic acid receptor (RAR) and retinoid X receptor (RXR) ligands together with pravastatin and fluconazole. Uptake of 1 μM estradiol-17β-glucuronide (containing 0.4 μCi/ml [3H]estradiol-17β-glucuronide) was measured at 37 °C for 20 s with OATP1B1-expressing and wild-type CHO cells in the absence or presence of inhibitors. Values obtained with wild-type CHO cells were subtracted from values obtained with OATP1B1-expressing CHO cells and are given as percent of the control. Values are given as the mean ± SD of triplicate determinations; * p < 0.05. Abbreviations: 5α-Androstane hexa: 5α-androstane-3β, 17β-diol 17-hexahydrobenzoate; HETE: 8(S)-hydroxy-(5Z,9E,11Z,14Z)-eicosatetraenoic acid.