Abstract
Background
The widespread use of HAART has led to marked decreases in death rates in Brazil in HIV-infected individuals. Nonetheless, there are scarce data on specific causes of death.
Methods
Death rates from a cohort of HIV-infected patients in Rio de Janeiro, Brazil were analyzed in two-year periods, from 1997 to 2006. Poisson models and survival models accounting for competing risks were used to assess association of co-variables. A standardized, validated algorithm was used to ascertain specific causes of death.
Results
Of the 1,538 eligible patients, 226 (14.7%) died during the study period, corresponding to a mortality rate of 3.2/100 person-years. The median follow-up time was 4.61 years (IQR = 5.63 years) and the loss to follow-up rate was 2.4/100 person-years. Overall, 98 (43.4%) were classified as non-AIDS-related causes. Although opportunistic infections were the leading causes of death (37.6%), deaths due to AIDS-related causes declined significantly over time (p<0.01). In the most recent period (2005-2006) the rate of non-AIDS related causes of deaths was higher than that of AIDS-related causes of death.
Conclusions
In the HAART era there has been a significant change in causes of death among HIV-infected patients in Rio de Janeiro. As access to HAART improves, integration with other public programs will become critically important for the long term success of HIV/AIDS programs in developing countries.
Keywords: HIV, AIDS, Brazil, causes of death
Introduction
In the past decade, the introduction and widespread use of highly active antiretroviral therapy (HAART) has led to marked decreases in mortality rates in low- and high-income countries 1-4, turning a deadly disease into a manageable chronic condition.
In developed countries, population-based and cohort studies have demonstrated that, although HIV/AIDS-related conditions remain the most frequent causes of death, other conditions, including diabetes mellitus (DM), cardiovascular diseases (CVDs), cancer, liver and renal diseases, have become increasingly frequent in HIV-infected individuals 5-10. In fact, in some populations, such as patients with CD4+ counts greater than 200 cells/mm3, non-HIV-related causes of death are now more frequent than HIV-related ones 1.
Due to the increased survival, the HIV-infected population tends to become older 11, a factor that in itself may contribute to the increased frequency of non-HIV/AIDS-related causes of death. An association between certain HAART regimens, particularly those containing protease inhibitors or abacavir, and risk of CVD and conditions such as the metabolic syndrome has been reported 12-14. A possible contributing role of uncontrolled HIV replication on the risk of non-HIV-related deaths has also been suggested 15.
In Brazil, where free access to HAART has been universal for all patients who qualify for treatment under locally developed guidelines since 1996, mortality rates among HIV-infected patients decreased sharply after the introduction of HAART, but have remained stable after 1999 3, 411} Recently, based on data from the national databases of death certificates, we reported a significant increase between 1999 and 2004 in non-HIV-related conditions, including CVD and DM, as causes of death 16.
In this study, we describe changes in temporal trends of causes of death in a cohort of HIV-infected patients in Rio de Janeiro, Brazil.
Methods
Data sources
All patients included in the study were followed at Hospital Universitário Clementino Fraga Filho (HUCFF). The hospital HIV database was originally designed to validate the World Health Organization (WHO) HIV staging system and is described elsewhere 17. HUCFF is a participating site of the ART-LINC collaboration (http://www.ispm.ch/artlinc.html) 1, 18 and of CCASAnet (http://ccasanet.vanderbilt.edu), both of which are part of the IeDEA network (http://www.iedea-hiv.org). Patients who entered the cohort between 1997 and 2006, were aged 16 years or older, and had at least one follow-up visit were eligible for the present study. Laboratory data, including CD4 counts and viral load, are electronically transferred from the hospital database. Clinical data are abstracted from hospital charts immediately after consultations by trained data abstractors who fill out specially designed forms that contain all relevant fields and variables.
Vital status was ascertained either by abstracting information from medical charts or by linkage with the Rio de Janeiro Mortality Database, using a previously validated algorithm 19. Information from death certificates obtained through the linkage algorithm, which included primary and contributing causes of death, was used in the ascertainment of causes of death as described below. Patients were considered to be lost to follow-up if no vital status information was available for more than one year.
Ascertainment of causes of death
Causes of death were determined using the CoDe (Coding Causes of Death in HIV) protocol 20. Briefly, extensive data were collected from all available sources, including death certificates, medical records, autopsy reports, and information obtained from family members by a trained physician or health care provider. Two independent reviewers attributed causes of death based on the completed CoDE forms. If the attributed cause of death was the same for both reviewers, it was considered to have been established. If there was disagreement between the two reviewers, or both had coded the cause of death as unknown or unclassifiable, the case was referred to a third reviewer for final classification.
Causes of death were classified as AIDS-related or non-AIDS-related based on the presence or absence of an AIDS-defining condition, according to the Centers for Disease Control and Prevention Classification 21, and as a primary or contributing cause of death. All deaths with unknown primary and contributing causes for which at least one CD4 cell count was available within 6 months prior to the date of death were classified as AIDS-related if CD4 counts were < 200 cells/mm3 and as non-AIDS-related if ≥ 200 cells/mm3. Deaths that did not meet any of the above criteria were classified as of unknown cause.
Underlying causes of death, which are defined by WHO as “(a) the disease or injury which initiated the train of morbid events leading directly to death or (b) the circumstances of the accident or violence which produced the fatal injury” 22 were further categorized according to whether or not they were (a) AIDS-related (according to CDC definitions): opportunistic diseases; cancer; both cancer and opportunistic diseases; and undefined disease(s); or (b) non-AIDS–related: hepatitis/liver-related; infectious diseases; non-AIDS-related cancer; external causes; CVD; other diseases; and unknown.
A separate analysis using an alternative CoDe classification 20 was also performed. According to this definition, a death was considered to have been due to immunodeficiency-related causes if it had a CDC AIDS-defining condition as primary or contributing cause of death, if the CD4 cell counts closest to death was < 50 cells/mm3 or if was due to non-sudden causes and the CD4 cell counts closest to death was < 200 cells/mm3). According to this classification, a death was considered as non-immunodeficiency-related if none of the causes reported were a CDC AIDS-defining condition and the CD4 cell counts closest to death was ≥ 200 cells/mm3, or if the death was sudden with CD4 cell counts closest to death between 50 and 200 cells/mm3.
Statistical analysis
Descriptive statistics are presented for the variables considered comparing living patients with deceased patients due to AIDS-related, non-AIDS-related, and unknown causes of death. For continuous variables, non-parametric Kruskal-Wallis tests were used followed by pair-wise Wilcoxon tests with Holm's multiple comparisons correction; for discrete variables, overall Fisher exact tests were performed.
Two calendar year rates of AIDS- and non-AIDS-related causes of death and of CVD (according to the CoDe protocol) per 100 person-years (py) were calculated and compared over time to detect temporal changes. Poisson regression models were employed and correlation and overdispersion were handled by using quasi-Poisson corrections for variance estimation 23.
Survival analysis regression models were performed in the context of competing risks 24, 25. This approach makes it possible to account for differences between non-informational censoring due to study termination or independent losses to follow-up and informational censoring, when losses to follow-up are due to death by causes other than the one(s) studied, allowing for correct modeling of the main outcome and competing risk(s) 25-27. Classically, event-specific hazards are modeled separately for the event of interest and the competing risk, which tends to overestimate the cumulative mortality, since it considers that the competing event does not exist. Another problem with this approach is that the assumption of proportionality across covariates strata will usually be violated and inferences could be biased 26. A new approach proposed by Fine and Gray 28, 29 allows the estimation of the subdistribution hazards for each event, which takes into account the competing risks, allowing for unbiased estimation of the cumulative incidence functions (CIFs) and is implemented in the ‘cmprsk’ library 30 in the R software. Adjusted hazard ratios (aHR) of the subdistributions are reported for the models.
Variables analyzed included gender, age at start and at the end of observation or death, transmission group [heterosexual, men who have sex with men (MSM), intravenous drug users (IDU), other and unknown], baseline CD4 counts, use of HAART (both as a fixed or a time-varying variable) and use of PI-containing regimens.
All analyses were performed in R for Windows 31.
Institutional Review Board approval was obtained both from the Hospital Universitário Clementino Fraga Filho, where the cohort is being followed, and the University of Pittsburgh.
Results
Of the 1,633 potentially eligible patients, 95 (5.8%) females were excluded from the analysis because they were followed only during pregnancy. Of the 1,538 eligible patients, who contributed 7,037 person-years of follow-up, 226 (14.7%) were known to have died during the study period, yielding a mortality rate of 3.2/100 person-years. Eighty-two deaths (36.3%) were identified only through the linkage algorithm with the Rio de Janeiro Mortality Database; compared to the mortality rate before the linkage algorithm was applied (2.0/100 py), the retrieval of these cases represented a greater than 50% increase (difference = 1.2; 95%CI = 0.9,1.4; p < 0.01). The median follow-up time was 4.61 years (IQR = 5.63 years) and the loss to follow-up rate was 2.4/100 person-years.
Characteristics of all patients are described in Table 1. Compared to patients remaining in care, those lost to follow-up were younger, more likely to be female, on HAART and on a PI-based regimen, had higher baseline CD4 cell counts, and a shorter follow-up time (p < 0.05 for all).
Table 1.
General descriptions of variables analyzed
| TOTAL N=1538 | Alive n=1312 | Deceased n=226 |
P values* | LFU n=170 | |||
|---|---|---|---|---|---|---|---|
| AIDS-related n=111 | Non-AIDS–related n=98 | Unknown n=17 | |||||
| Variables | |||||||
| Age at start of follow-up in years, median (IQR) | 34 (15) | 33 (14) | 35 (13) | 36 (18) | 36 (15) | 0.02 | 30 (13) |
| Age at end of follow-up in years, median (IQR) | 39 (15) | 39 (15) | 38 (12) | 41 (15) | 37 (14) | 0.47 | 33 (12) |
| Female gender, n (%) | 772 (50.2) | 673 (51.3) | 46 (41.4) | 44 (44.9) | 9 (52.9) | 0.16 | 100 (59) |
| HIV transmission category | |||||||
| Heterosexual, n(%) | 761 (49.5) | 661 (50.4) | 45 (40.5) | 48 (49.0) | 7 (41.2) | Ref. | 75 (44.1) |
| MSM, n(%) | 249 (16.2) | 222 (16.9) | 16 (14.4) | 6 (6,1) | 5 (29.4) | 0.04 | 30 (17.6) |
| IDU, n(%) | 52 (1.8) | 40 (1.1) | 5 (2.7) | 6 (9.2) | 0 (0) | 0.13 | 1 (0.59) |
| Other, n(%) | 52 (3.4) | 40 (3.0) | 5 (4.5) | 6 (6.1) | 1 (5.9) | 0.13 | 6 (3.5) |
| Unknown, n(%) | 449 (29.2) | 374 (28.5) | 42 (37.8) | 29 (29.6) | 4 (23.5) | 0.16 | 58 (34.1) |
| Baseline CD4 in cells/μL, median (IQR) | 250 (338) | 268 (337) | 95 (272)# | 148 (299)# | 182 (277) | <0.01 | 340 (336) |
| HAART ever, n (%) | 1158 (75.3) | 998 (76.0) | 77 (69.4) | 72 (73.5) | 11 (64.7) | 0.26 | 94 (55.3) |
| HAART during observation period, n (%) | 1032 (67.1) | 879 (67.0) | 75 (67.6) | 67 (68.4) | 11 (64.7) | 0.98 | 96 (56.5) |
| PI ever, n (%) | 774 (50.3) | 650 (49.5) | 54 (48.6) | 62 (63.3) | 8 (47.1) | 0.07 | 117 (68.8) |
| Outcomes | |||||||
| Death rate per 100 PY | 3.21 | 1.58 | 1.39 | 0.24 | 0.37 | - | |
| PY of observation, median (IQR) | 4.6 (5.6) | 5.0 (5.8) | 1.5 (3.1)# | 3.4 (4.6)#§ | 3.5 (3.5) | <0.01 | 2.6 (3) |
Overall p-values for Kruskal-Wallis test for continuous and Fisher exact test for discrete variables, including alive and unknown patients. For rates, mid-p exact Poisson tests were used, comparing non-AIDS and AIDS deaths only.
Pair-wise p-value < 0.05 comparing with alive patients
Pair-wise p-value < 0.05 comparing with the competing cause
Ref – Reference group
PY – person-years
Of the 226 known deaths, 111 (49.1%) were classified as due to AIDS-related, and 98 deaths (43.4%) as due non-AIDS-related causes; 17 (7.5%) were due to unknown causes. Patients who died from non-AIDS related conditions contributed more follow-up time than patients who died from AIDS-related conditions (median time = 3.39 py vs. 1.45 py, respectively, p < 0.01) (Table 1). Rates of death from AIDS-related causes (1.58/100 py) and from non-AIDS-related causes (1.39/100 py) were not statistically different (p = 0.37).
Alive patients had higher baseline CD4 cell counts than deceased patients in pairwise comparisons with both patients who died from AIDS- and from non-AIDS-related causes of death (p < 0.01 for both comparisons). The difference in baseline CD4 counts between the latter two groups did not reach statistical significance after multiple comparisons adjustment (148 vs. 95 cells/mm3, respectively, p = 1).
Causes of death are shown in Table 2. Opportunistic infections were the leading causes of death (37.6%). Among non-AIDS-related causes, infectious diseases were also the most frequent causes (8.4%), followed by external causes (4.9%) and CVD (4.0%).
Table 2.
Groups of specific causes of death
| Groups | N | % |
|---|---|---|
| AIDS-related | ||
| Opportunistic | 85 | 37.6 |
| Cancer | 17 | 7.5 |
| Both | 2 | 0.9 |
| Other | 7 | 3.1 |
| Non-AIDS-related | ||
| Hepatitis/Liver | 8 | 3.5 |
| Infectious | 19 | 8.4 |
| Cancer | 8 | 3.5 |
| External | 11 | 4.9 |
| CVD | 9 | 4.0 |
| Other | 43 | 19.0 |
| Unknown | 17 | 7.5 |
| Total | 226 | 100 |
CVD – Cardiovascular diseases
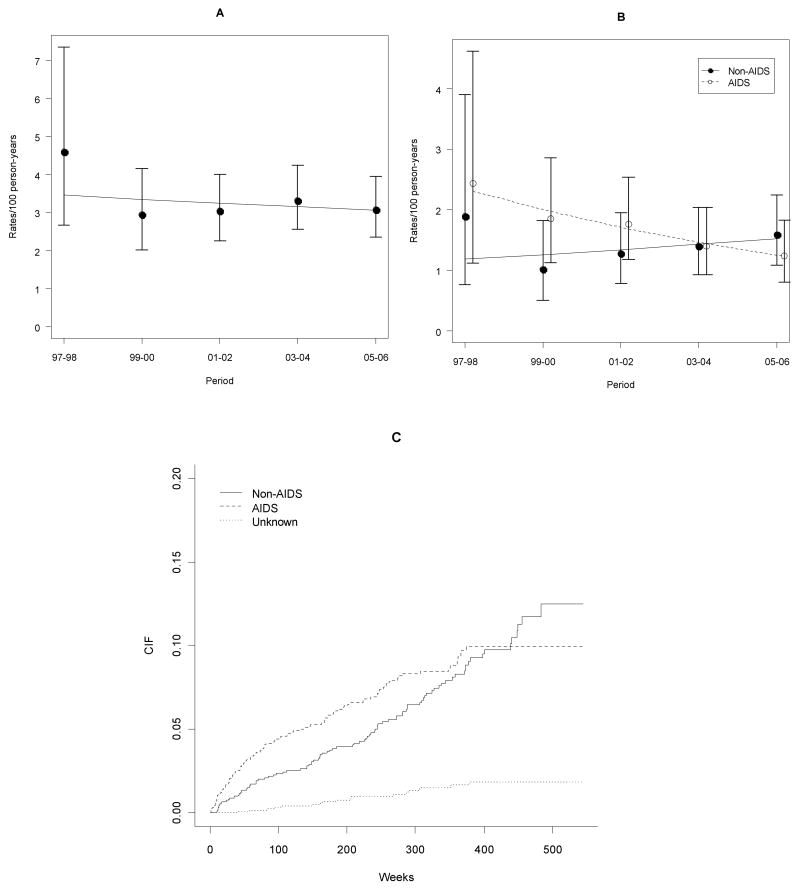
Overall death rates remained fairly stable during the study period (p = 0.57). In contrast, AIDS-related causes of death declined significantly over time (p < 0.01), and non-AIDS-related causes of death increased over time, although not significantly (p = 0.46) (Table 3 and Figure 1). Nonetheless, in the most recent period analyzed (2005-2006) the rate of non-AIDS related causes of deaths was higher than that of AIDS-related causes of death (1.59/100 py and 1.24/100 py, respectively). CVD as an underlying cause of death increased over time, although the increase did not reach statiscal significance (p = 0.09) (Table 3).
Table 3.
Trends of overall, AIDS-related causes, non-AIDS-related causes and CVD death rates per 100 person-years over time and 95% confidence intervals for all patients
| 1997-1998 | 1999-2000 | 2001-2002 | 2003-2004 | 2005-2006 | p trend | |
|---|---|---|---|---|---|---|
| Total | 4.59 (2.68,7.36) | 2.96 (2.02,4.17) | 3.04 (2.25,4.0) | 3.32 (2.56,4.24) | 3.08 (2.36,3.95) | 0.57 |
| AIDS | 2.43 (1.11,4.62) | 1.85 (1.13,2.85) | 1.76 (1.18,2.53) | 1.4 (0.92,2.04) | 1.24 (0.80,1.83) | <0.01 |
| Non-AIDS | 1.89 (0,76,3.90) | 1.02 (0.51,1.82) | 1.28 (0.79,1.95) | 1.4 (0.92,2.04) | 1.59 (1.09,2.24) | 0.46 |
| CVD | 0 (0,1.0) | 0 (0,0.34) | 0.06 (0.01,0.34) | 0.21 (0.06,0.53) | 0.20 (0.20,0.51) | 0.09 |
CVD – Cardiovascular diseases considered as underlying cause of death
Figure 1.
Temporal trends of deaths in the cohort. A – Overall death rates and 95% CIs and linear trend over time (Poisson model); B – AIDS and Non-AIDS-related death rates and 95% CIs and linear trend over time (Poisson model); C – Cumulative incidence functions (CIFs) of the subdistributions of deaths associated with AIDS-, non-AIDS-related and unknown causes in a competing risks framework.
The cumulative incidence functions for the hazards subdistributions for AIDS-related, non-AIDS-related, and unknown causes of death in a competing risks framework are shown in Figure 1C. The cumulative incidence of AIDS-related causes of death was higher than non–AIDS-related causes of death at the beginning of the observation period, with a reversal of this pattern during the subsequent seven years. In Table 4 we present models for the hazard subdistributions of AIDS- and non-AIDS related causes of death, with age at the start of the observation period as a time-dependent variable, adjusted for baseline CD4 cell count, gender, and HIV transmission category. The models indicate that higher baseline CD4 cell counts were strongly associated with protection for both AIDS- and non-AIDS-related causes of death (10% and 13% per 50 cells increase, respectively) and that patients in the intravenous drug use transmission category were at higher risk of dying of a non-AIDS-related cause of death (aHR = 4.96; 95% CI: 2.34,10.52; p < 0.01), but not of an AIDS-related cause of death. Although baseline age was not associated with non-AIDS-related causes of death, there was a 46% increase in risk per 10 years of baseline age for AIDS-related causes of death, with a significant and negative interaction over time (p = 0.02), indicating that risk decreased significantly over time.
Table 4.
Models for the hazards of the subdistributions and specific-hazards of deaths due to AIDS- and to non-AIDS-related causes including baseline age (per 10 year increase), CD4 cell counts (per 50 cells increase), gender (reference: female) and risk group (IDU vs. other), and an interaction term between baseline age and log of time under observation. Baseline age treated as a time-dependent variable.
| Outcome | Variables | aHR (95% CI) | p |
|---|---|---|---|
| Deaths due to AIDS-related causes | Baseline Age (10 years) 1 | 1.46 (1.15,1.85) | <0.01 |
| Baseline CD4 (50 cells) | 0.87 (0.82,0.92) | <0.01 | |
| Male gender | 1.07 (0.71,1.59) | 0.75 | |
| IDU | 1.35 (0.42,4.31) | 0.61 | |
| Deaths due to non-AIDS-related causes | Baseline Age (10 years) 2 | 1.30 (0.94,1.80) | 0.12 |
| Baseline CD4 (50 cells) | 0.90 (0.86,0.95) | <0.01 | |
| Male gender | 0.84 (0.56,1.26) | 0.40 | |
| IDU | 4.96 (2.34,10.52) | <0.01 | |
Negative interaction with time (p = 0.02)
Negative interaction with time (p = 0.77)
aHR: Adjusted hazard ratio
HAART and PI use during the observation time were not significantly associated with non-AIDS-related causes of death (HR = 0.7; 95%CI: 0.44,1.09; p = 0.11 and HR = 1.23; 95%CI: 0.82,1.86; p = 0.32, respectively).
Death rates due to causes not related to immunodeficiency increased significantly over time (0.54 vs. 0.84 deaths per 100 py, in 1997-1998 and 2005-2006, respectively; p < 0.01), even though death rates due to causes related to immunodeficiency were consistently and significantly higher throughout the study period (data not shown).
Eight cases that lacked data on the causes of death were classified as AIDS-related based solely on the CD4 cell count 6 months prior to death. A sensitivity analysis recoding these cases as unknown did not change the results (data not shown).
Discussion
Our results show that overall mortality rates in a large cohort of HIV-infected patients in Rio de Janeiro, Brazil, remained fairly stable between 1999 and 2006. This is in agreement with other studies that showed that in Brazil, although mortality rates declined sharply after HAART became widely available, they have remained stable since 1999 4, 16. In our study, when AIDS- and non-AIDS-related causes of death were examined separately, a decrease in AIDS-related causes of death and an increase in non-AIDS-related causes of death were observed. When more restrictive criteria were used to classify causes of death, non-immunodeficiency related causes of death increased significantly over time.
Even though the temporal trend for non-AIDS-related causes of death did not reach statistical significance, in the most recent study period (2005-2006) non-AIDS-related causes of death became more frequent than AIDS-related causes of death. Despite the small number of cases, there was an increase of CVD as a cause of death during the study period, even though it did not reach statistical significance (p = 0.09). Thus, the distribution and temporal trends for specific groups of causes of death we encountered are in agreement with reports from developed countries, in which although opportunistic infections remain the main cause of death, non-AIDS-related causes of death are becoming increasingly common 1, 7.
In a regression model for the hazards subdistribution adjusted for possible confounders, although baseline age was significantly associated with risk for AIDS-related causes of death, the risk significantly decreased over time. On the other hand, baseline age had no significant effect on the risk for non-AIDS-related causes of death. These results indicate that, as patients age because of longer survival times, they become at higher risk of dying from a non-AIDS-related condition. These findings are in accordance with the recent description of HIV cohorts tending to get older 11, as is the case in our cohort, where the median age significantly increased from 33.25 years in 1997 to 39.0 years in 2006 (p < 0.01).
Our results are in agreement with several reports in the literature that have documented changes in causes of death among HIV-infected patients in the HAART era, with decreases in AIDS-related causes of death, and increases of cancer, cardiovascular and liver diseases 5-10. For example, using combined information on HIV/AIDS surveillance with vital statistics data from New York City, Sackoff et al. 6 showed an increase in the proportion of non-HIV/AIDS-related causes of death from 19.8% to 26.3% between 1999 and 2004, with substance abuse, CVD and cancer as the top ranking conditions. In the HIV Outpatient Study, Palella et al. 8 showed that non-AIDS-related causes of death increased from 13.1% to 42.5% between 1996 and 2004, with CVD being the leading cause of death in this group in 2004. More recently, Lewden et al 9 showed significant increases in cancer, liver diseases and CVD as non-AIDS-related causes of death in HIV-infected adults in France and Novoa et al 10 showed similar trends in Spain. We have recently shown that in Brazil between 1999 and 2004 the proportion of non-AIDS-related causes of death reported on death certificates of patients that had HIV/AIDS as one of the causes of death increased significantly, as did CVD 16.
Among the main strengths of our study was the use of a database linkage algorithm that allowed us to determine the vital status of patients who otherwise would be considered to be lost to follow-up, which increased the death rate by 57% and kept the loss to follow-up rate at 2.4/100 person-years. Even though vital status was still missing for 170 patients lost to follow-up, we believe this has not affected our results, as these patients tended to be younger and to have higher CD4 cell counts than patients remaining in the cohort. Thus, if these losses to follow-up were due to death, the likely cause is a non-AIDS-related condition, as these characteristics are associated with non-AIDS-related causes of death.32, 33 Another strength of our study was the use of the CoDe algorithm 20, which allowed for a standardized classification of causes of death, which would not be possible if only data abstracted from death certificates or medical charts were used.
The main limitation of our study was the relatively small number of deaths (n = 226), which made some of the models we used underpowered for associations. Another limitation was the retrospective nature of the study, which did not allow us to investigate potentially important variables, such as adherence to treatment, smoking status, hepatitis co-infection, and lipid and cholesterol blood levels.
In conclusion, our results indicate that in the HAART era there has been a significant change in causes of death among HIV-infected patients in Rio de Janeiro, Brazil. Although overall death rates remained stable after 1999, deaths from AIDS-related causes decreased, while deaths from non-AIDS-related causes increased to the point that the latter became more common than the former. The major driving force behind this change appears to be longer survival due to the significant decrease in deaths due to AIDS-related causes. Our findings have major programmatic implications for developing countries. As access to HAART improves and HIV becomes a chronic condition, integration with other public programs, such as smoking cessation and control of hypertension will become critically important for the long term success of HIV/AIDS programs in developing countries.
Acknowledgments
Financial support: This study was partially supported by the Fogarty International Center, National Institutes of Health (3 D43 TW01038 to the University of Pittsburgh) and a Research Career Development Award, National Institute of Allergy and Infectious Diseases (K24 AI52788 to Dr. Harrison)
References
- 1.Braitstein P, Brinkhof MW, Dabis F, et al. Mortality of HIV-1-infected patients in the first year of antiretroviral therapy: comparison between low-income and high-income countries. Lancet. 2006 Mar 11;367(9513):817–824. doi: 10.1016/S0140-6736(06)68337-2. [DOI] [PubMed] [Google Scholar]
- 2.Palella FJ, Jr, Delaney KM, Moorman AC, et al. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998 Mar 26;338(13):853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Brito AM, Castilho EA, Szwarcwald CL. Regional patterns of the temporal evolution of the AIDS epidemic in Brazil following the introduction of antiretroviral therapy. Braz J Infect Dis. 2005 Feb;9(1):9–19. doi: 10.1590/s1413-86702005000100004. [DOI] [PubMed] [Google Scholar]
- 4.Marins JR, Jamal LF, Chen SY, et al. Dramatic improvement in survival among adult Brazilian AIDS patients. Aids. 2003 Jul 25;17(11):1675–1682. doi: 10.1097/00002030-200307250-00012. [DOI] [PubMed] [Google Scholar]
- 5.Crum NF, Riffenburgh RH, Wegner S, et al. Comparisons of causes of death and mortality rates among HIV-infected persons: analysis of the pre-, early, and late HAART (highly active antiretroviral therapy) eras. J Acquir Immune Defic Syndr. 2006 Feb 1;41(2):194–200. doi: 10.1097/01.qai.0000179459.31562.16. [DOI] [PubMed] [Google Scholar]
- 6.Sackoff JE, Hanna DB, Pfeiffer MR, Torian LV. Causes of death among persons with AIDS in the era of highly active antiretroviral therapy: New York City. Ann Intern Med. 2006 Sep 19;145(6):397–406. doi: 10.7326/0003-4819-145-6-200609190-00003. [DOI] [PubMed] [Google Scholar]
- 7.Smit C, Geskus R, Walker S, et al. Effective therapy has altered the spectrum of cause-specific mortality following HIV seroconversion. Aids. 2006 Mar 21;20(5):741–749. doi: 10.1097/01.aids.0000216375.99560.a2. [DOI] [PubMed] [Google Scholar]
- 8.Palella FJ, Jr, Baker RK, Moorman AC, et al. Mortality in the Highly Active Antiretroviral Therapy Era: Changing Causes of Death and Disease in the HIV Outpatient Study. J Acquir Immune Defic Syndr. 2006 Sep;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16. [DOI] [PubMed] [Google Scholar]
- 9.Lewden C, May T, Rosenthal E, et al. Changes in causes of death among adults infected by HIV between 2000 and 2005: The “Mortalite 2000 and 2005” surveys (ANRS EN19 and Mortavic) J Acquir Immune Defic Syndr. 2008 Aug 15;48(5):590–598. doi: 10.1097/QAI.0b013e31817efb54. [DOI] [PubMed] [Google Scholar]
- 10.Novoa AM, de Olalla PG, Clos R, Orcau A, Rodriguez-Sanz M, Cayla JA. Increase in the non-HIV-related deaths among AIDS cases in the HAART era. Curr HIV Res. 2008 Jan;6(1):77–81. doi: 10.2174/157016208783572017. [DOI] [PubMed] [Google Scholar]
- 11.Casau NC. Perspective on HIV infection and aging: emerging research on the horizon. Clin Infect Dis. 2005 Sep 15;41(6):855–863. doi: 10.1086/432797. [DOI] [PubMed] [Google Scholar]
- 12.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003 Nov 20;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 13.d'Arminio A, Sabin CA, Phillips AN, et al. Cardio- and cerebrovascular events in HIV-infected persons. Aids. 2004 Sep 3;18(13):1811–1817. doi: 10.1097/00002030-200409030-00010. [DOI] [PubMed] [Google Scholar]
- 14.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007 Apr 26;356(17):1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 15.El-Sadr WM, Lundgren JD, Neaton JD, et al. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006 Nov 30;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- 16.Pacheco AG, Tuboi SH, Faulhaber JC, Harrison LH, Schechter M. Increase in non-AIDS related conditions as causes of death among HIV-infected individuals in the HAART era in Brazil. PLoS ONE. 2008;3(1):e1531. doi: 10.1371/journal.pone.0001531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schechter M, Zajdenverg R, Machado LL, Pinto ME, Lima LA, Perez MA. Predicting CD4 counts in HIV-infected Brazilian individuals: a model based on the World Health Organization staging system. J Acquir Immune Defic Syndr. 1994 Feb;7(2):163–168. [PubMed] [Google Scholar]
- 18.Dabis F, Balestre E, Braitstein P, et al. Cohort Profile: Antiretroviral Therapy in Lower Income Countries (ART-LINC): international collaboration of treatment cohorts. Int J Epidemiol. 2005 Oct;34(5):979–986. doi: 10.1093/ije/dyi164. [DOI] [PubMed] [Google Scholar]
- 19.Pacheco AG, Saraceni V, Tuboi S, et al. Validation of a Hierarchical Deterministic Record Linkage Algorithm Using Data From Two Different Cohorts of HIV-Infected Individuals and Mortality Databases in Brazil. Am J Epidemiol. 2008;168(11):1326–1332. doi: 10.1093/aje/kwn249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.CoDe. Coding Causes of Death in HIV. [Jan-25-2007];2007 www.cphiv.dk/CoDe.
- 21.CDC. From the Centers for Disease Control and prevention. 1993 revised classification system for HIV infection and expanded surveillance case definition for AIDS among adolescents and adults. Jama. 1993 Jan 27;269(4):460. [PubMed] [Google Scholar]
- 22.WHO. International Statistical Classification of Diseases and Health Related Problems ICD-10, Volume 2: Instruction Manual. 2nd. 2004. [Google Scholar]
- 23.Agresti A. Categorical data analysis. 2nd. New York: Wiley-Interscience; 2002. [Google Scholar]
- 24.Glynn RJ, Rosner B. Comparison of risk factors for the competing risks of coronary heart disease, stroke, and venous thromboembolism. Am J Epidemiol. 2005 Nov 15;162(10):975–982. doi: 10.1093/aje/kwi309. [DOI] [PubMed] [Google Scholar]
- 25.Therneau TM, Grambsch PM. Modeling survival data : extending the Cox model. New York: Springer; 2000. [Google Scholar]
- 26.Putter H, Fiocco M, Geskus RB. Tutorial in biostatistics: competing risks and multi-state models. Stat Med. 2007 May 20;26(11):2389–2430. doi: 10.1002/sim.2712. [DOI] [PubMed] [Google Scholar]
- 27.Gaynor JJ, Feuer EJ, Tan CC, et al. On the Use of Cause-Specific Failure and Conditional Failure Probabilities: Examples From Clinical Oncology Data. J Am Stat Assoc. 1993;88(422):400–409. [Google Scholar]
- 28.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. [Google Scholar]
- 29.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16(3):1141–1154. [Google Scholar]
- 30.cmprsk: Subdistribution Analysis of Competing Risks. [computer program]. Version. 2006. [Google Scholar]
- 31.R: A language and environment for statistical computing. [computer program] Version 2.4.0. Vienna, Austria: R Foundation for Statistical Computing; 2006. [Google Scholar]
- 32.Lau B, Gange SJ, Moore RD. Risk of Non-AIDS-Related Mortality May Exceed Risk of AIDS-Related Mortality Among Individuals Enrolling Into Care With CD4+ Counts Greater Than 200 Cells/mm3. J Acquir Immune Defic Syndr. 2007 Oct 30;44(2):179–187. doi: 10.1097/01.qai.0000247229.68246.c5. [DOI] [PubMed] [Google Scholar]
- 33.Monforte A, Abrams D, Pradier C, et al. HIV-induced immunodeficiency and mortality from AIDS-defining and non-AIDS-defining malignancies. AIDS. 2008 Oct 18;22(16):2143–2153. doi: 10.1097/QAD.0b013e3283112b77. [DOI] [PMC free article] [PubMed] [Google Scholar]