Table 1.
Investigation of Palladium Precatalysts and Reoxidants
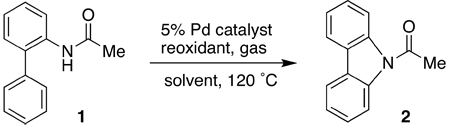 | ||||||
|---|---|---|---|---|---|---|
| Entry | Pd source | Reoxidant /equiv |
Solvent | Gas atm |
Time (h) |
Yield (%)a |
| 1 | Pd(OAc)2 | Cu(OAc)2 / 1 | toluene | O2 | 12 | 92 |
| 2 | Pd(OAc)2 | toluene | O2 | 12 | 5 | |
| 3 | Cu(OAc)2 / 1 | toluene | O2 | 12 | 0 | |
| 4 | Pd(O2CCF3)2 | Cu(OAc)2 / 1 | toluene | O2 | 24 | 70 |
| 5 | PdCl2 | Cu(OAc)2 / 1 | toluene | O2 | 24 | 21 |
| 6 | PdCl2(CH3CN)2 | Cu(OAc)2 / 1 | toluene | O2 | 24 | 27 |
| 7 | PdCl2(PPh3)2 | Cu(OAc)2 / 1 | toluene | O2 | 24 | 67b |
| 8 | Pd(OAc)2 | PPh3 / 0.2 | toluene | O2 | 12 | 5 |
| 9 | Pd(OAc)2 | pyridine / 0.2 | toluene | O2 | 12 | 1 |
| 10 | Pd(OAc)2 | PhI(OAc)2 / 0.2 | DCEc | air | 12 | 25 |
| 11 | Pd(OAc)2 | K2S2O8 / 5 | DCEd | air | 12 | 2 |
| 12 | Pd(OAc)2 | benzoquinone / 1 | toluene | air | 12 | 4 |
GC yield was determined with respect to a calibrated internal standard.
About 5–10% diacetylated amide of 2-aminobiphenyl was observed.
Slightly higher yield was obtained in dichloroethane than in methylene chloride.
The reaction was performed at 80 °C in dichloroethane; the same low yield was observed with or without bases.
