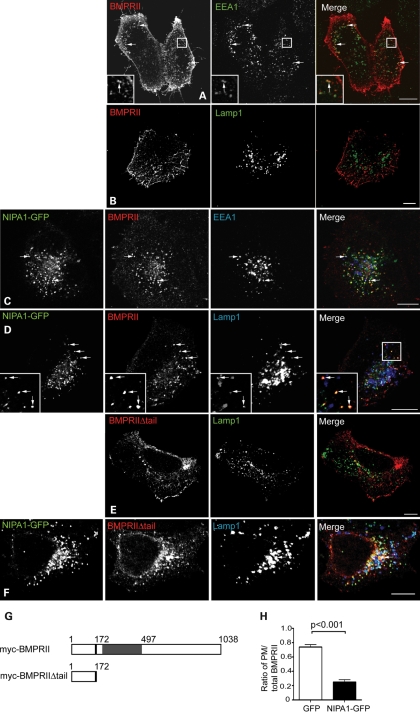
Figure 3.
NIPA1 promotes traffic of BMPRII to a lysosomal compartment in HeLa cells. Wild-type HeLa cells (A, B and E) or cells stably expressing NIPA1-GFP (C, D and F) were transiently transfected with myc-BMPRII (A–D) or myc-BMPRIIΔtail (E and F) and labelled with the early endosomal marker EEA1 (A and C) or the lysosomal marker Lamp1 (B and D–F). In the absence of NIPA1, there is minimal co-localization between BMPRII and EEA1 (A) and almost no co-localization between BMPRII and Lamp1 (B and E). In the cells expressing NIPA1-GFP (C, D and F), note the striking internalization of both full-length and truncated BMPRII from the plasma membrane, the appearance of co-localization between NIPA1-GFP and BMPRII (which appears yellow in the merged images) and of three-way co-localization between NIPA1, BMPRII and EEA1/Lamp1. In (A), (C), (D) and (F), arrows indicate discrete puncta showing co-localization. (G) Schematic diagram illustrating the two N-terminal myc-tagged constructs used in experiments. The constructs encoded either full-length BMPRII (myc-BMPRII) or a truncated form of BMPRII lacking the intracellular tail (myc-BMPRIIΔtail). The transmembrane region is shown in black, with the serine/threonine kinase domain in grey. (H) Representative experiment showing quantitation of plasma membrane anti-myc-BMPRII fluorescence versus total BMPRII fluorescence, in HeLa cells transiently expressing GFP alone (32 cells), or stably expressing NIPA1-GFP (35 cells). Error bars: SEM. P-value was calculated using unpaired t-test.